Figures & data
Figure 1. Overall strategy for the generation of tailor-made cytokine mimetics based on sdAb-derived bispecifics and YSD-enabled antibody discovery.
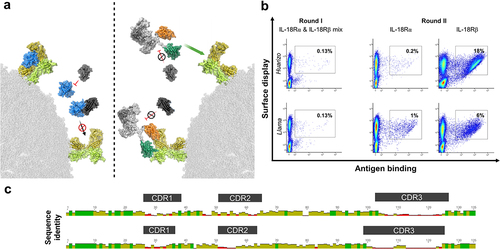
Figure 2. Combinatorial reformatting of monospecific (1 + 0) SEEDbodies into strictly monovalent (1 + 1) bsAbs enables the identification of IL-18 mimetics with attenuated capacities to trigger NFκB reporter activity on IL-18 reporter cells.
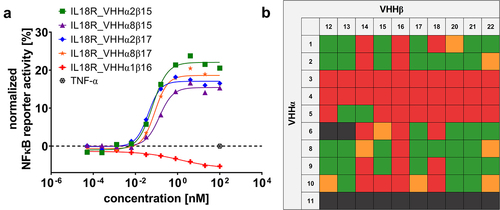
Figure 3. Bispecific (1 + 1) surrogate agonists trigger IFN-γ release on PBMCs isolated from healthy donors.
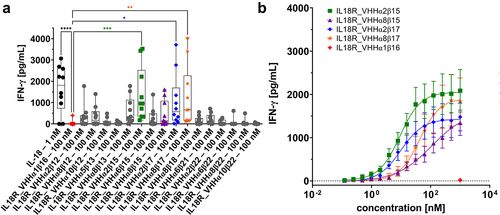
Table 1. Binding kinetics and functional properties of the herein generated four leading bispecific IL-18 mimetics.
Figure 4. Antibody Engineering enables the generation of IL-18 mimetics with augmented agonism capacities.
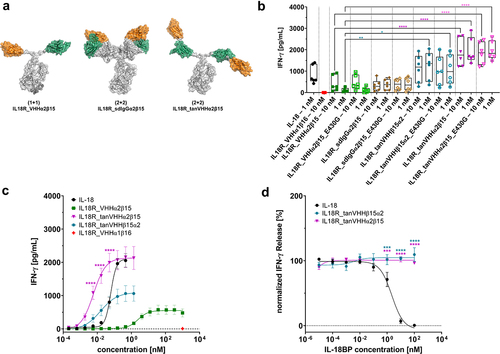
Table 2. Biophysical, biochemical, and functional attributes of engineered cytokine mimetic formats.
