Figures & data
Figure 1. Proof-of-concept microfluidic functional screening for in-trans TNFRSF agonism or autocrine target-specific internalization. (a) CHO cell lines (red) are pre-labeled and express either a cancer related antigen (CRA) in parallel to secretion of an anti-CRA-anti-TNFRSF bispecific antibody or a non-targeted anti-HEL isotype bispecific antibody (not illustrated). A TNFRSF member on engineered Jurkat cells can be activated in trans to produce a green signal via GFP that can be sorted for. (b) microfluidic sorting of a 1:100 mixed CRA-targeted to non-targeted red pre-labeled antibody secreting cells for green reporter signal. Note unspecific background signal below the sorting gate of droplets lacking secreting cells but containing leaky and/or multiple reporter cells. (c) Statistics for throughput, PCR recovery and hit rates. (d) Scheme of exemplary anti-B7-H3 reference antibody m276 binding to and internalizing with recombinant B7-H3 in autocrine fashion. pH-dependent detection antibody signal can be harnessed to sort for internalization in red peak versus average mode. (e) Cyto-Mine sort plot of 1:100 m276:anti-HEL secreting cell mixture for internalization signal and resulting hit rate. (f) High PCR recovery rates indicate robustness of CHO cells; high m276 hit rate indicates robustness of the autocrine assay setup.
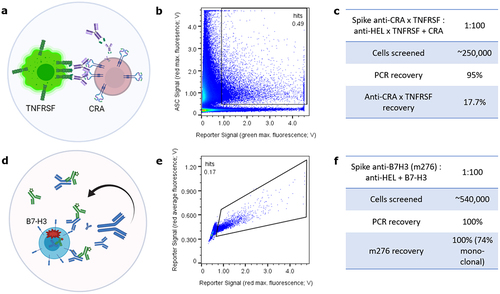
Figure 2. Inducible antibody display with soluble Protein a addition retains genotype-phenotype correlation and differential display allows sorting for good manufacturability properties. (a) analytical flow cytometry analysis of 1F11 ZZ-PDGFR-TMD CHO cells displaying CS06 antibody (lambda) or bococizumab (kappa) in a 1:10 mixture after 24 h co-cultivation mimics a library situation. Considerable amounts of cells were positive for both lambda and kappa antibody display in Q2 due to paracrine background signal of bococizumab on CS06-displaying cells. (b) setup as in (a) with addition of soluble Protein a as decoy for paracrine antibodies restores exclusive display of autocrine produced antibody and generates a genotype-phenotype coupling sufficient for sorting. (c) differential induced CHO surface display levels of cetuximab (light blue) versus bococizumab (red) of approximately 3-fold as indicated by normalized MFI values. Uninduced non-displaying cells as reference are shown in orange. (d) Individual stains of differential display of CS06 antibody (blue) versus bococizumab (red) indicate the option to sort for manufacturability in a 1:100 mixture (orange) applying the indicated gate. Analytical lambda versus kappa flow cytometry analyses of this 1:100 mixture input (e) and sort output (f) indicate enrichment of CS06 in Q1 from approximately 1% to 10%. Events in Q3 represent bococizumab, while non-display-induced cells are observed in Q4.
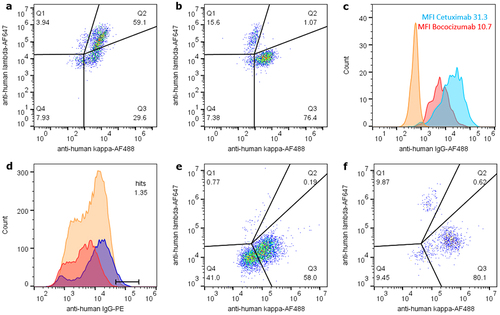
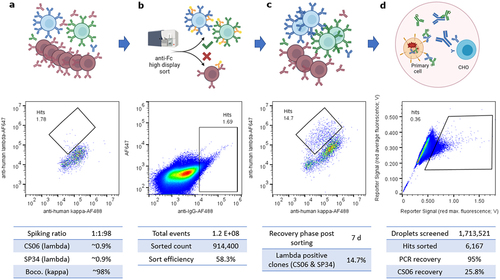
Figure 3. Integrated sorting for manufacturability and internalization via display to secretion switching. (a) a defined 1:1:98 mixture of 1F11 ZZ-PDGFR-TMD CHO cells secreting anti-c-MET lambda antibody CS06 (blue), anti-CD3 lambda SP34 (green) or anti-PCSK9 kappa antibody bococizumab (red) was incubated for 24 h prior to additional 24 h display induction to mimic potential cross-contamination of undesired paracrine library antibodies, here exemplarily bococizumab. (b) the induced clone mixture was sorted for high display via anti-IgG-AF488 and cells were recovered by cultivation without doxycycline induction for 7 d. (c) a split of sort output was then induced for analytical lambda versus kappa flow cytometry evaluation as indicator for enrichment of clones with good versus poor manufacturability properties. (d) Finally, the non-induced sort output was co-encapsulated with c-MET positive EBC-1 tumor cells and screened for c-MET-specific CS06 internalization using the Cyto-Mine microfluidic device. 6167 events were sorted and 192 dispensed. 48 of 186 analyzed single dispensed droplets yielded a CS06 specific PCR amplicon, equaling 25.8% hit rate. Enrichment of CS06 in this two-step screening approach was therefore approximately 30-fold.