Figures & data
Figure 1. Screen for hybridoma-derived mAbs with hPRL-neutralizing activity. (a) hPRLR-expressing CHOK1SV GS-KO cells were stimulated with increasing concentrations of hPRL (0–434 nM), hGH (0–1,010 nM), or hPL (0–1,449 nM) for 15 minutes at 37°C before measuring hPRLR signaling by phospho-STAT5 TR-FRET activity assay. Data was normalized to maximum response observed for each agonist. Values represent mean ± SEM of two (hPL) or three independent experiments (hPRL and hGH). (b) hPRLR-expressing CHOK1SV GS-KO cells were stimulated with 20 nM hPRL preincubated with 100 nM of various hybridoma-derived mAbs or control mAbs with reported PRL-neutralizing activity. Data was normalized to maximum response of untreated stimulated cells. Values represent mean ± SEM of two independent screens. The dotted horizontal line represents 80% inhibition cutoff.
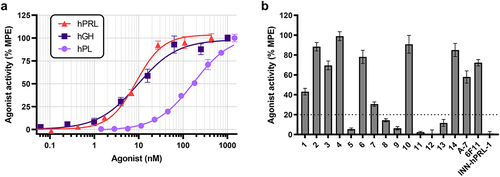
Figure 2. Binding kinetics of PL 200,039. Biolayer interferometry analysis of the binding of PL 200,039 to (a) hPRL, (b) hGH, (c) hPL, (d) nhpPRL, and (e) rPRL accomplished by immobilizing PL 200,039 onto anti-human-Fc capture biosensors, and incubating a range of concentrations (100, 33, 11 nM or 45, 15, 5 nM) of each analyte. Kinetic parameters were calculated using a 1:1 model with global fitting. Experimental response at each concentration are shown with each calculated fitted curve (solid lines).
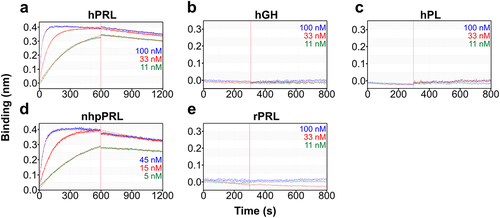
Table 1. Kinetic rate constants and equilibrium dissociation constants for the binding interactions of lactogenic hormones with PRL mAbs. If binding was not observed, rates are labeled as not detected (n.d.).
Figure 3. Selectivity and cross-reactivity of PL 200,039. (a) hPRLR-expressing CHOK1SV GS-KO cells were stimulated for 15 minutes at 37°C with constant 20 nM hPRL, 20 nM hGH, or 350 nM hPL preincubated with varying concentrations (0–150 nM or 0–2.6 µM) of PL 200,039 for 30 minutes prior to stimulation. Data was compiled from three independent experiments and normalized to response of cells stimulated in the absence of antibody. Values represent mean ± SEM. (b) CHOK1SV GS-KO cells expressing hPRLR or mPRLR were stimulated as before with constant 20 nM nhpPRL or 20 nM rPRL, respectively, preincubated for 30 minutes with varying concentrations (0–150 nM) of PL 200,039 prior to stimulation. Data was compiled from two (rPRL) or three (nhpPRL) independent experiments and normalized to the response of cells stimulated in the absence of antibody. Values represent mean ± SEM.
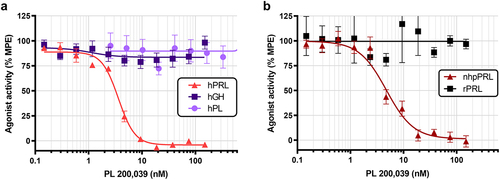
Table 2. Inhibition of PRLR signaling by PRL mAbs.
Table 3. Competitive binding of PRL mAbs to FcγRs by immunoassay.
Figure 4. Pharmacokinetic profile of PL 200,031 and PL 200,039 after single dose (5 mg/kg) IV administration in FcRn-humanized Tg32 mice. Data represents mean serum concentrations ± SD from each group (n = 3).
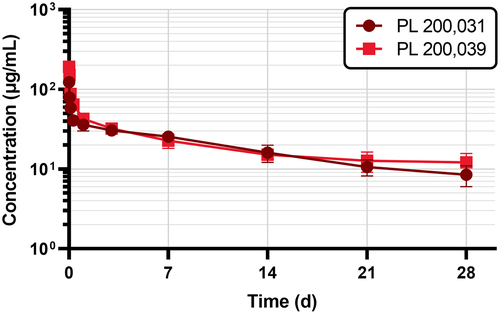
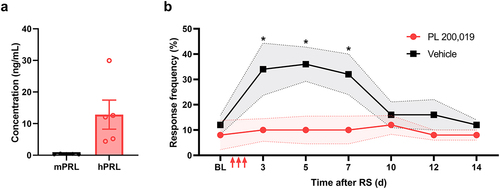
Figure 5. Treatment with PL 200,019 blocks stress-induced mechanical hypersensitivity in female PRL-humanized mice. (a) measurement of PRL in five female PRL-humanized mice using species-specific ELISA. Data presented as mean value for each animal and overall mean ± SEM. (b) stress-induced mechanical hypersensitivity measurement in female PRL-humanized mice treated with PL 200,019 (20 mg/kg) or vehicle (n = 5 per group). Baseline mechanical sensitivity (BL) was measured prior to two hours of restraint stress (RS) repeated for three consecutive days (marked by arrows). Data presented as mean ± SEM. *p < 0.05 (two-way ANOVA).