Figures & data
Figure 1. Sampling schemes for a comparison of manual and automatic sampling of OTA in barley. Figures illustrate the positions of incremental samples in the container. (a) Increments during the monitoring of OTA formation where open and filled circles indicate aggregate samples 1 and 2, respectively. (b) End-point manual sampling where aggregate samples are formed from incremental samples collected at positions 1–8. (c) Formation of eight aggregate samples by interpenetrating automatic sampling.
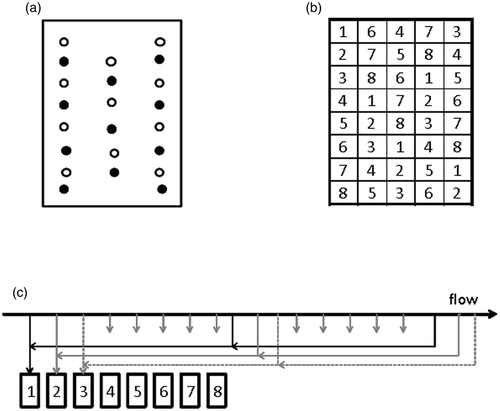
Figure 2. OTA estimations in barley, experimental design and procedures for sampling and sample preparation. Aggregate samples were formed by manual and automatic sampling. After sample splitting, milling and reduction, four test samples were obtained in the manual sampling procedure and two test samples in the automatic sampling, respectively. Each test sample was analysed twice for OTA concentration by means of immunoaffinity clean up and HPLC-FLD. Brackets indicate total uncertainty (u t) and uncertainty from sampling with automatic (u as) or manual (u ms) sampling, sample reduction (u r), subsampling (u sub1) and analysis (u a) (analysis = subsampling2 + extraction + HPLC).
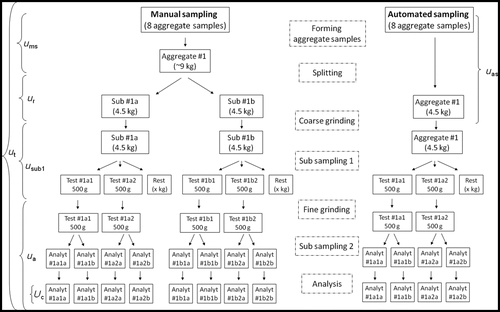
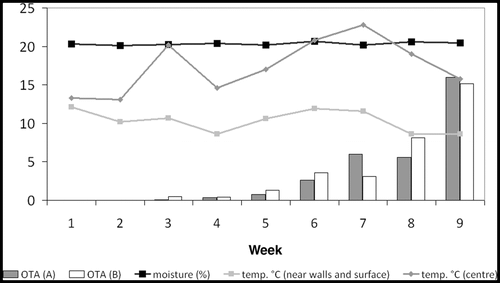
Figure 3. Monitoring of temperature, moisture content and concentration of OTA (µg kg−1) in barley inoculated with Penicillium verrucosum at ambient temperature. Bars represent OTA concentration of aggregate samples (A, B), respectively. Graphs indicate the temperature (°C) near the surface and in the centre of the lot and the average moisture content (%).