Figures & data
Table 1. A brief description of the blinded 9 cereal test samples
Table 2. Blinded, non-guided testing of 9 different cereal samples in 5 different external laboratories using 10 different extraction methodologies reveals a lack of agreement on the levels of oestrogenic activity present (in pg oestradiol equivalents/g cereal)
Figure 1. The oestrogenic activities of repeated oestradiol standard curves vary between assays indicating the relative sensitivity and reproducibility of each test system. The CALUX and STTA mammalian cell models were most sensitive, followed by the A-YES and YES yeast assays. All 4 models reproducibly produced similar concentration-response relationships in subsequent tests on subsequent days. This is not particularly surprising as all 4 tests have been vetted by ISO (A-YES and YES) or OECD (CALUX and STTA) after formal interlaboratory validation studies (OECD Citation2012, Citation2016, Citation2017; ISO Citation2018a, Citation2018b). Lab 5’s unvalidated OED model was both the least sensitive and the least reproducible of all the tests. For comparison, we have also provided the oestradiol standard curves collected using the CALUX assay in our own lab over the same timeframe as the external study
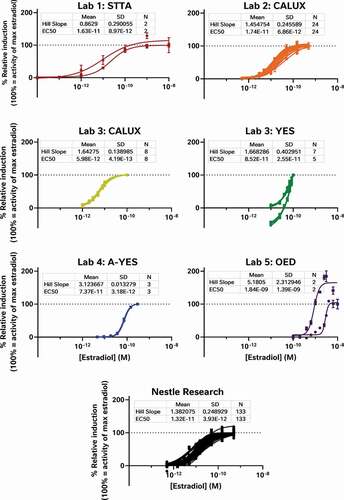
Figure 2. The consequence of diverse extraction and test methodologies is conflicting results for increasing concentrations of different breakfast cereal extracts. Methodology varied: (red) acidified methanol – STTA, (light orange) ASE with acetone – CALUX, (medium orange) ASE with ethanol – CALUX, (dark orange) methanol – CALUX, (yellow) acidified methanol – CALUX, (green) acidified methanol – YES, (blue) acidified methanol – A-YES and (purple) acetone – OED; closed symbols and solid lines indicate no deconjugation step, while extracts with open symbols and dashed lines were deconjugated.) Differing oestrogenic (A-I) and cell viability activities (J-R) were observed for the same cereal sample; whereas different samples prepared using the same methodology produced similar results
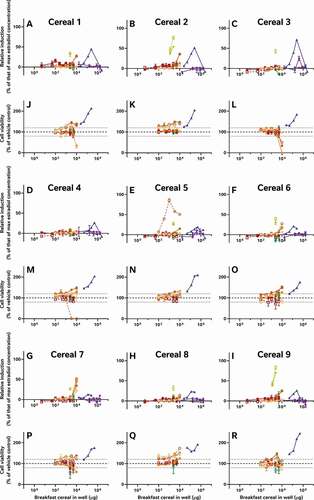
Figure 3. Methodologies for sample extraction and testing affect measured estrogenicity more than the sample identity. The consequence of diverse extraction and test methodologies is conflicting oestrogenic activities of breakfast cereals. The numbers within each cell indicate the measured oestrogenic activity, represented as a fold-change over experimental LOQ (or 5 %RI, whichever is greater), such that activities can be compared between different assays. These values are then reflected in a heatmap in which the lowest activities are coloured blue, fold-changes of 1 (equal to the LOQ) are grey and the maximum estrogenicity observed is red. These are amalgamated with the findings from expert judgment, the shape of which codes for (![]()
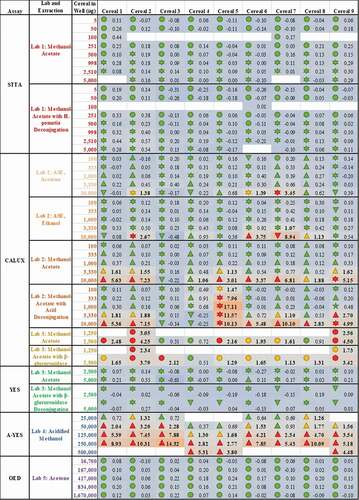
Figure 4. An optimised, fit-for-purpose alkaline extraction protocol (blue) is needed to release and deconjugate the matrix-bound phytoestrogens in cereals, but co-treatment with a fixed concentration of oestradiol (![]()
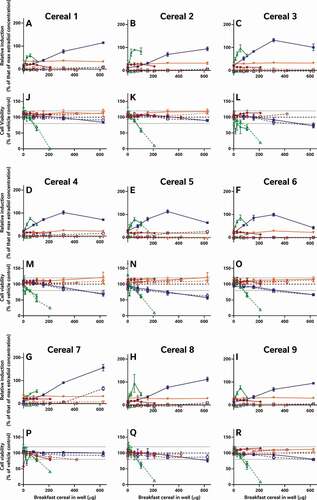
Figure 5. The method of sample extraction affects measured estrogenicity more than sample identity; even when the test lab and bioassay used are standardised, the measured oestrogenic activities vary more as a result of the chosen extraction method (rows) than the identity of the sample (columns). The consequence of this finding is conflicting results for the oestrogenic activity of breakfast cereals. As before, the numbers within each cell indicate the measured oestrogenic activity, represented as a fold-change over experimental LOQ (or 5% RI, whichever is greater); these are translated into a heatmap in which the lowest activities are coloured blue, fold-changes of 1 (equal to the LOQ) are grey and the maximum estrogenicity observed is red. The findings from expert judgment are also shown where the shape codes for viability![]()
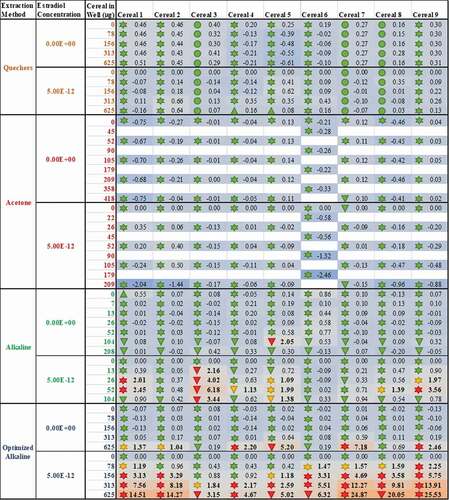
Figure 6. Alkaline extraction methods, but not acetone and QuEChERS were able to recover the oestrogenic activity of spiked-in genistein from either a process blank (blue) or whole wheat flakes (red). The unoptimised alkaline method was able to extract the oestrogenic activity of 280 nM genistein, it was cytotoxic to the cells at 3 of the 4 concentrations tested. Thus, neither the acetone, QuEChERS nor the unoptimised alkaline extraction method was considered suitable for the extraction of the breakfast cereals. Only the optimised alkaline method extracted the genistein without inducing more than mild cytotoxicity at the top concentration tested. Genistein-spiked samples are presented as dark colours and unspiked extracts as lighter versions; percent relative induction is shown using closed symbols and solid lines, while percent relative viability has open symbols with dashed lines
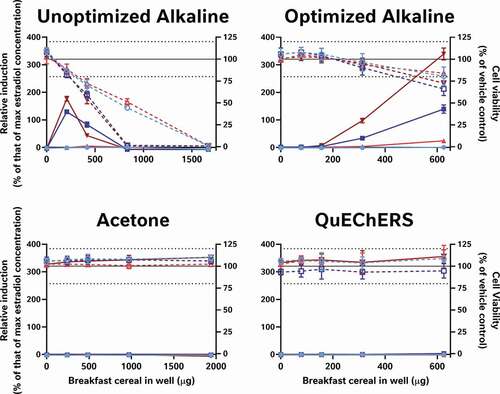
Figure 7. Optimised alkaline extraction recovered the oestrogenic activity from spiked breakfast cereals. To demonstrate the recovery of this activity, varying amounts of genistein were spiked into milled whole wheat flakes or sham experiments at different levels in a matrix design. These samples were then extracted to generate a genistein concentration-response curve at every cereal test level for both a process blank (Panel A; from lightest to darkest blue: vehicle control, 78.1, 156, 313, or 625 µg) or whole wheat flakes (Panel B; from lightest to darkest red: vehicle control, 78.1, 156, 313, or 625 µg cereal). In both panels, superinduction of oestrogenic activity was observed from genistein treatment at all cereal levels. (Cell viability data were also collected for these experiments, but for clarity are not shown here.) In a follow up experiment (Panels C and D), these results were compared with those from unspiked process blank and extract of whole-wheat flakes which had been spiked post-extraction with the genistein concentration-response curve at the 625 µg cereal level (shown in muted colours and dotted lines). These tests indicate that the alkaline extraction process transforms the genistein into a more active oestrogenic substance of unknown identity (Panel C). Cell viability (Panel D, dashed lines) is however, unchanged
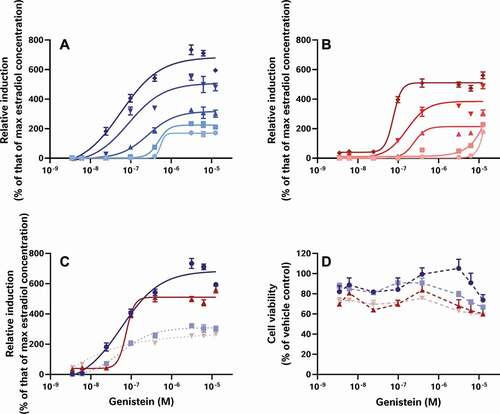
Figure 8. Three independent replicates of the optimised alkaline extraction method (in varying shades of blue) had similar oestrogenic activities in the CALUX assay (here shown as percent relative induction without further processing). These data indicate that both the extraction and bioassay methods were reproducible. However, the activities measured were low; consequently, the optimised alkaline extracts had to be ‘boosted’ into the dynamic range of the bioassay by co-treatment with a fixed 5 pM concentration of oestradiol (Panels A-![]()
Figure 9. ‘Boosting’ the optimised alkaline extracts into the dynamic range of the CALUX assay by co-treatment with a fixed 5 pM concentration of oestradiol can result in oestrogenic activity in the process blanks (Panel A;![]()
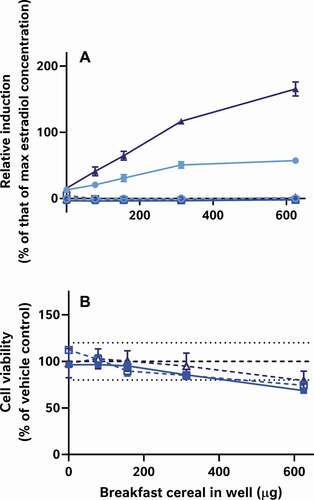
Figure 10. The matrix-bound phytoestrogens in cereals can be semi-quantified using oestrogen activity measures; however, it is necessary to subtract both the 5 pM oestradiol ‘boost’ (where applicable) and the concurrent process blank from the measured oestrogenic activities (Panels A-I). The resulting blank-subtracted relative inductions estimate the part of the measured oestrogenic activities which can be ascribed solely to the tested cereal samples in the ‘boosted’ (![]()
Table 3. Oestradiol-equivalencies of the optimised alkaline extracts reveal the oestrogenic activities of these 9 cereal samples
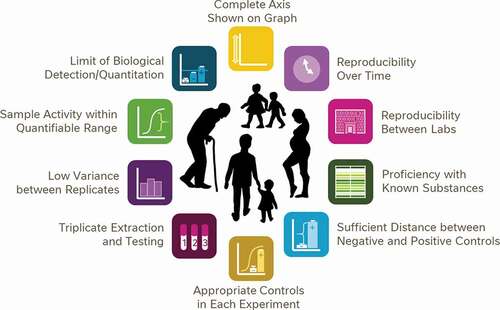
Figure 11. A proposed framework of 10 simple points for consumers and non-experts to consider when assessing the suitability of data for the purposes of decision making or consumer advice
Supplemental Material
Download Text (1,011.8 KB)Supplemental Material
Download Text (41 KB)Supplemental Material
Download Text (532 KB)Supplemental Material
Download Text (984.5 KB)Supplemental Material
Download Text (938.2 KB)Supplemental Material
Download MS Word (55.4 KB)Data availability statement
Nestlé Research holds a license from BioDetection Systems (Amsterdam, Netherlands) for the use of the CALUX assay. All other materials used are widely available. The authors declare that all raw data supporting the findings of this study are available within the paper and its supplementary information files. Spotfire data analysis files are available from the corresponding author upon reasonable request.
