Figures & data
Table 1. Prevalence of adulterated herbal products with active pharmacological ingredients.
Table 2. LC–MS/MS-based levels of individual compound(s) in supplements, their total concentrations, recommended daily doses and EDIs.
Table 3. Comparison between estimated concentrations in the PDE-Glo assay and the LC–MS/MS analysis. Only supplements classified as resulting in medium or high intake based on the bioassay are shown.
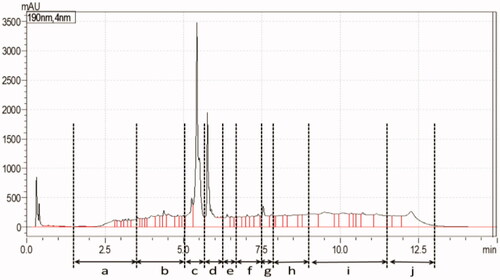
Figure 2. Inhibition potentials of collected fractions of S13 tested in the PDE-Glo bioassay, fraction c showing the presence of a PDE-5i.
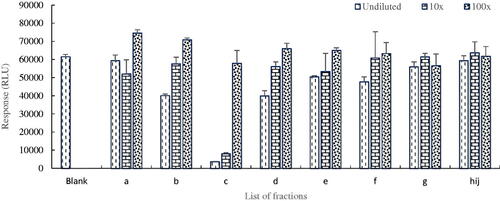
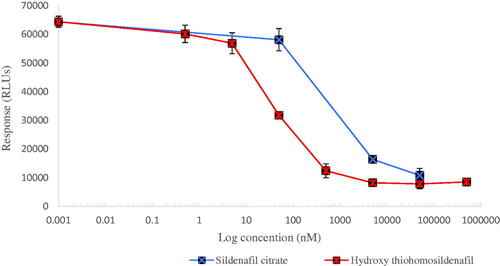
Figure 3. Concentration-dependent reduction in PDE-5 activity. IC50 values of 900 nM and 80 nM were calculated from the fitted dose–response curves for sildenafil and hydroxythiohomosildenafil, respectively, resulting in a REP value for hydroxythiohomosildenafil of about 12.