Figures & data
Table 1. The Box-Behnken experimental design to formulate nanosuspensions (NSps) as carriers of acetogenins (βCDSL-ACGs-NSps) with the testing and predicted response variables.
Table 2. Analysis of variance and regression coefficients of polynomial quadratic models predicted with the formulation conditions of acetogenin-loaded nanosuspensions on particle size.
Figure 1. Response surface plots of the particle size from nanosuspension loaded with acetogenins obtained with low agitation (500 rpm) (a), High agitation (1000 rpm) (b), ultrasound, (c) and pareto plot (d). XCD, hydroxypropyl-β-cyclodextrin (%); XSL, soy lecithin (mg/mL); XPM, dispersion method.
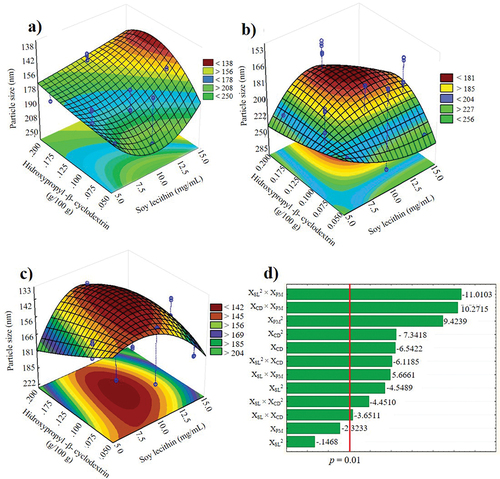
Table 3. Optimal experimental conditions to formulate nanosuspensions as carriers of acetogenins (βCDSL-ACGs-NSps) and experimental validation.
Figure 2. Fourier-transform infrared spectroscopy (a), X-ray diffraction (b), and ultraviolet-visible diffuse reflectance spectroscopy (c) of isolated acetogenins (ACGs) and the optimized nanosuspension loaded with acetogenins (OβCDSL-ACGs-NSps) optimized nanosuspension loaded with acetogenins. βCD-SL complex, hydroxypropyl-β-cyclodextrin complexed with soy lecithin.
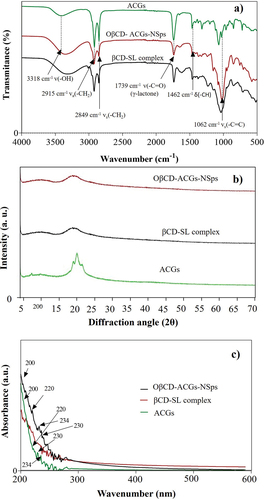
Figure 3. Particle size distribution of the optimized nanosuspension loaded with acetogenins (OβCD-ACGs-NSps)(a), particle morphology by atomic force microscopy of OβCD-ACGs-NSps (b), and 3D micrograph of OβCD-ACGs-NSps (c).
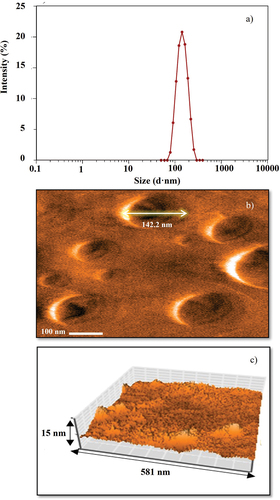
Figure 4. Representative HPLC-chromatogram of acetogenins extracted from optimized nanosuspension (OβCD-ACGs-NSps).
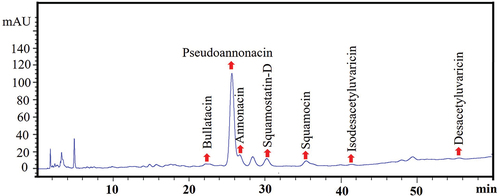
Figure 5. Cumulative release of ACGs from the optimized nanosuspension (OβCD-ACGs-NSps) and prediction of the overall release behavior according to the Korsmeyer-Peppas model (a), bacterial inhibition of E. faecalis (b) and L. monocytogenes (c) of OβCD-ACGs-NSps. k, release constant; n, release exponent; R2, coefficient of determination; DMSO, Dimethylsulfoxide; βCD-SL, hydroxypropyl-β-cyclodextrin complexed with soy lecithin; antibiotic, trimethoprim with sulfamethoxazole (3200 μg/mL).
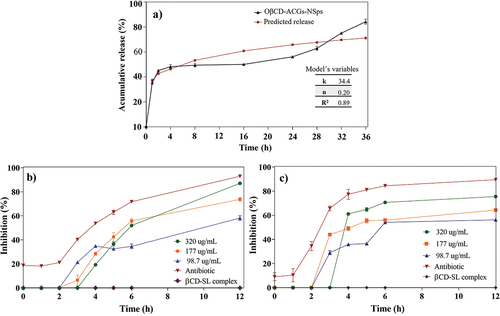
Table 4. Acgs release kinetics for zero-order, Higuchi and Korsmeyer-Peppas model and n-value.
