Figures & data
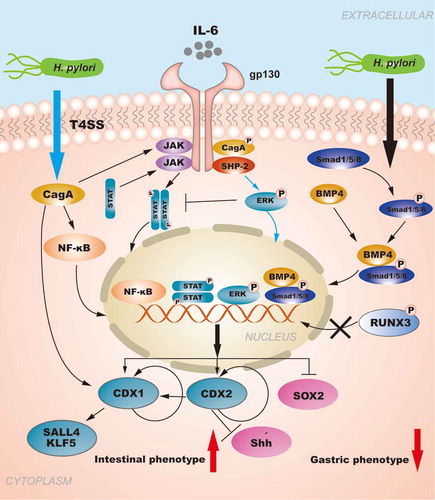
Figure 1. The regulation of CDXs in GIM induced by NF-κB signaling pathway and pro-inflammatory cytokines. H. pylori injects the oncoprotein CagA into gastric epithelial cells using a type IV secretion system (T4SS) to activate the NF-κB signaling pathway. NF-κB activation induces the release of the pro-inflammatory cytokine IL-6, and IL-6 binds to its specific receptor gp130, activating two major signaling pathways: SHP-2/ERK and JAK/STAT. CagA can also affect the signal transduction of gp130 regulation, and the resulting biological effect depends on the tyrosine phosphorylation status of CagA. Moreover, the SHP2/ERK and JAK/STAT signaling pathways are considered to play opposite roles in gastric epithelial cells.
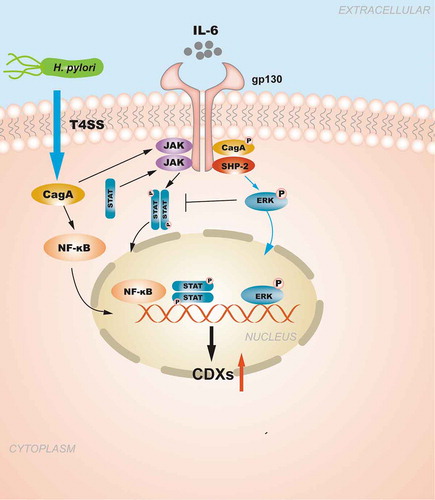
Figure 2. The regulation of CDXs in GIM induced by Transforming growth factor-beta (TGF-β) signaling pathway. H. pylori infection promotes the phosphorylation of SMAD1/5/8 and forms a complex with SMAD4, the complex is further transferred from the cytoplasm to the nucleus to regulate the transcription of CDX2. RUNX3 is also involved in the regulation of CDXs, abnormal cytoplasmic localization of RUNX3 caused by phosphorylation prevents it from exerting biological effects.
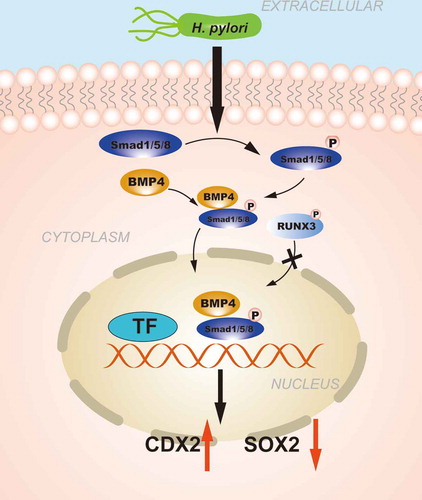
Figure 3. The role of CDXs in GIM: The regulation of CDXs in GIM induced by H. pylori. H. pylori injects the oncoprotein CagA into gastric epithelial cells using a type IV secretion system (T4SS) to activate the NF-κB signaling pathway. NF-κB activation induces the release of the pro-inflammatory cytokine IL-6, and IL-6 binds to its specific receptor gp130, activating two major signaling pathways: SHP-2/ERK and JAK/STAT. CagA can also affect the signal transduction of gp130 regulation, and the resulting biological effect depends on the tyrosine phosphorylation status of CagA. Moreover, the SHP2/ERK and JAK/STAT signaling pathways are considered to play opposite roles in gastric epithelial cells, and SHP-2/ERK signaling plays a greater role in inhibiting proliferation and inducing cell differentiation in the gastric mucosa. H. pylori infection can activate the BMP pathway to upregulate CDX2 expression concomitantly with SOX2 downregulation. RUNX3 is also involved in the regulation of CDXs. Furthermore, the autoregulation of CDXs maintains their expression in GIM. In summary, H. pylori infection is responsible for increasing the levels of intestinal-specific transcription factors and decreasing the levels gastric-specific transcription factors, which contribute to the development of GIM.