Figures & data
Figure 1. Examples of mode of action of probiotic adhesion factors. The figure shows binding and downstream effects on intestinal epithelial cells (IECs) and immune cells, reported for some probiotics with characterized adhesins. Probiotic pili-mediated adhesion favors a better colonizationCitation34 (1a), direct interaction to the intestinal mucus glycoproteinsCitation35 (1b), increased expression of tight junction-encoding genesCitation36 (1c) and direct interaction to antigen-presenting cells (dendritic cells)Citation37 (1d). SLPs-mediated adhesion through SLPs on bacteria or isolated SLPs occurs through interaction with TLR receptors on IECs (2a) and immune cells (macrophages)Citation23,Citation38,Citation39(2b) possibly this sustains production of fecal IgACitation40(2c). Studies using bacterial mutants of either SLPs or SLPs co-localized molecules show that SLPs trigger antigen-presenting cells (dendritic cells) inducing pro-inflammatory (IL-12, TNF-α, IL-1β) and anti-inflammatory cytokines (IL-6, IL-10)(2d).Citation41–43 Probiotic EPSs-mediated adhesion directly on other bacteria and/or competition for common adhesion sites on the host promotes “competitive exclusion” of pathogens or interferes with probiotic adhesion (3a).Citation44,Citation45 EPSs were shown to protect from innate immune mechanism via AMPs the probiotic LGG after adhesion to IECsCitation46 (3b). PSA on B. fragilis or isolated PSA has been shown to inhibit IL-1β induced inflammation through interaction with TLR2 and 4 on IECsCitation47,Citation48 (4a) and can also directly bind to antigen-presenting cells (dendritic cells) triggering downstream immune responsesCitation49,Citation50(4b). Distinct adhesins in the bacteria cells, not belonging to any of the aforementioned class, could support the sustained effects of L. casei on IgA and calprotectin Citation51–54(5). LGG probiotic triggers immediate adaptive immune response via B cells in humans.Citation13 Although the responsible upstream adhesion factors are not known the immediate effect is most possibly due to adhesion factors (6). The illustrative figure is shown without subdivision of GIT location (i.e. small or large intestine) but regional differences in the GIT immune system are of utmost importance.Citation55 In addition, the figure does not represent all mechanisms investigated by the studies in this review or literature but is meant to illustrate examples. Abbreviations: IECs, intestinal epithelial cells; PSA, polysaccharide A; SLPs, surface layer proteins; EPS, exopolysaccharides; IL, interleukin; IgA, immunoglobulin A; LGG, Lacticaseibacillus rhamnosus GG; AMPs, antimicrobial peptides. Created with BioRender.com.
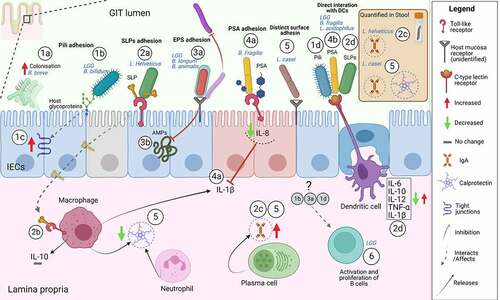
Table 1. Modifications of adhesion structures affects the probiotic function. Specific modification of surface structures on probiotics, hypothesised to be potential adhesins, affected GIT rewiring with respect to direct interaction, immune stimulation through inflammatory cytokines production and pathogens inhibition.
Figure 2. Age and probiotics adhesion. The figure shows a schematic view of probiotic and GIT characteristics changing with age. (a) Conventionally probiotics are given in increasing doses with age.Citation184 (b) IECs, which are among the main cells interacting with probiotics in the GIT and form the intestinal barrier function, are less established early in life but with age they are progressively shaped into a fully functioning GIT barrier.Citation10,Citation188 Probiotic adhesion is strain- and age-dependent. In (c) are shown a few examples of probiotic strains, B. bifidum,Citation189 LGG,Citation10,Citation189 B. lactis Bb12Citation189, in which age-dependency has been investigated within a single study. Created with BioRender.com
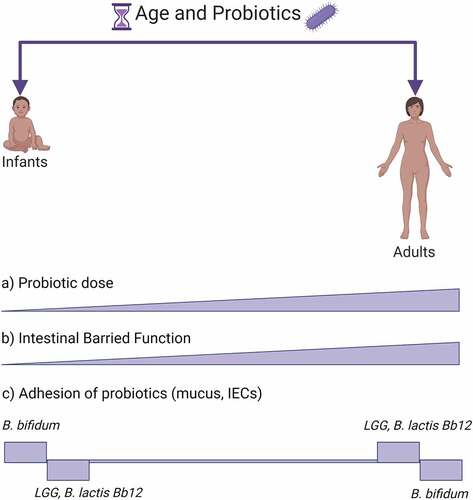
Figure 3. Key adhesion factors as potential proxy for a global probiotic function. Representation chart of some adhesion factors and potential application for global mechanisms with respective reference studies. First column: EPSs on LGG,Citation44–46 B. animalisCitation63 and B. breve.Citation67 Second column: PSA on B. fragilis.Citation47–50 Third column: Pili on B. bifidumCitation36,Citation93 and LGG.Citation37,Citation106,Citation108,Citation109 Fourth column: SLPs and SLAPs on L. acidophilus.Citation41–43,Citation115 Fifth column: distinct adhesins on L. plantarumCitation123,Citation141 and L. reuteri.Citation131 Abbreviations: EPS, exopolysaccharides; LGG, L. rhamnosus GG; PSA, polysaccharide A; SLPs, surface layer proteins; SLAPs, surface layer associated proteins; Cbp, collagen binding protein; Mub, mucus-binding protein. Created with BioRender.com
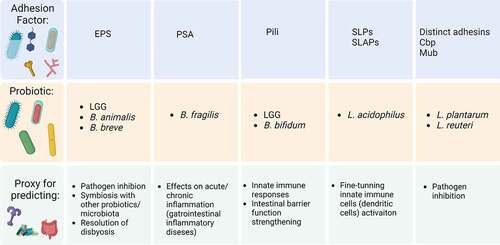
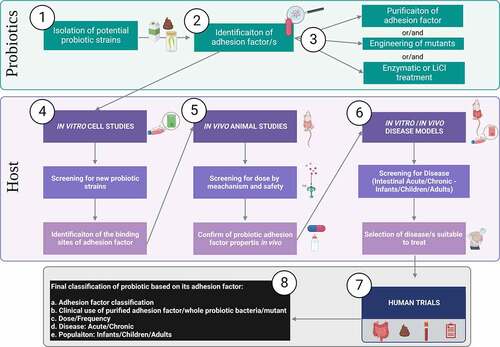
Figure 4. Ideal step-by-step workflow for studies of adhesion factors role in probiotic function. Given the mixed type of approaches found in literature the figure summarizes an ideal workflow for screening, experimental testing, and classification of probiotics considering adhesion factors. Bacteria from dairy, fermentation of fecal matter, etc, are isolated as potential probiotics (Step 1). Based on the bacteria type a hypothetical adhesion factor should be identified (Step 2). Then follows the purification of the adhesion factor (e.g. proteinic, polysaccharidic component), generation of mutants lacking, overexpressing the adhesion factor or enzymatic treatment of the bacteria (Step 3). In vitro studies, using most often IECs (e.g. Caco-2 cells) are the first experimental model used to investigate bacteria and adhesion factors for probiotic properties. Cell studies at this stage should ideally investigate the host receptors involved in the adhesion mechanism (Step 4). Once a probiotic and its adhesion mechanism have been identified, animal studies must aim to propose a dose range and mechanism of action in vivo (Step 5). Although adhesion factors are most often inert proteinic or complex carbohydrate by nature, hence considered safe, they could potentially be considered as drug-like when studying the metabolism in vivo to understand their half-life in the body. Once a preliminary hypothesis of the adhesin and healthy host is formulated, the next step should involve studies to decide which disease could benefit (Step 6). For instance, if it was hypothesized that an adhesion factor could benefit inflammatory conditions, established inflammatory models should be used to test this. Such models are for instance inflammation induced in IECs Caco-2 cell with inflammatory cytokines (e.g. IL-1β, TNF-α)Citation47 or/and animal models such as dextran sulfate sodium (DSS)-induced colitis in animals.Citation11 Next step comprises human trial conduction (Step 7). Although conduction of human trials in a stepwise manner takes longer, when possible, they should be performed in the following order: starting from pilot testing in healthy to study mainly safety, pilot trial in disease to mainly select a dose, and finally RCTs, Cross-Over or Parallel design trials with specific health outcomes. The 4 symbols represent samples used during human trials, intestinal biopsies, fecal samples, blood samples and symptoms questionnaires that can be used depending on the endpoint investigated and feasibility. Finally, considering both the probiotic and the host an adhesion factor-driven classification can be assigned to the probiotic (Step 8).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
