Figures & data
Figure 1. The growth profiles (left) and estimated doubling times (hours, right) of Faecalibacterium prausnitzii strains A2-165 (a), AHMP21 (b), and KLE1255 (c) using either the basal medium alone (i.e. M2G broth, denoted by the gray areas) or M2G broth supplemented with 0.1% (w/v or v/v) of different food additives (annotated according to the color-based key). In the panels left, only polysorbate 80 (black) and sodium sulfite (yellow) have profound effects on the growth profiles for all three F. prausnitzii strains. In contrast, the other food additives elicit minimal effects on the growth profiles of F. prausnitzii strain A2-165 and strain KLE-1255, but appeared to impose some burden on the growth profile of F. prausnitzii strain AHMP21. In the panels right, only the doubling time of F. prausnitzii strain KLE1255 was significantly increased by the presence of saccharin (P ≤ .001). The growth profiles are plots of mean OD600 (± SD) values obtained from two rounds of cultivation, each in technical triplicate (n = 6). Doubling times and statistical comparisons were made from the same data, as described in the Methods section.
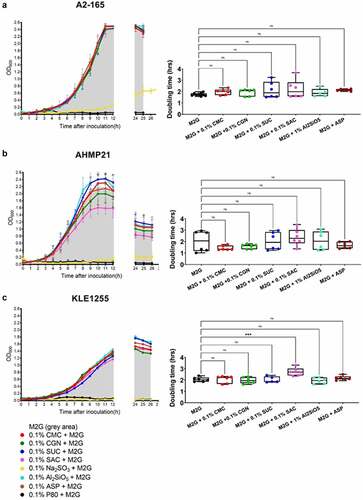
Figure 2. Polysorbate 80 and sodium sulfite appear to exert a bacteriostatic effect on the growth of Faecalibacterium prausnitzii strains A2-165 (a), AHMP21 (b) or KLE1255 (c). Here, the OD600 of cultures was monitored for 8 hours after either polysorbate 80 (black) or sodium sulfite (yellow) was added to cultures of the F. prausnitzii strains during their exponential phase of growth. Cultures receiving either sterile anaerobically prepared water (gray) or no additions (red) were also maintained, to substantiate that any effects on growth were food additive-dependent. Exposure to sodium sulfite strongly arrested the growth of all three F. prausnitzii strains for the time course of the study, and while the magnitude of the effect from polysorbate 80 differed among the three strains, in all instances both the growth rate and maximum yield were reduced. Panel D shows the results of separate experiments where subsamples of cultures as described above were used to inoculate tubes of M2G broth medium and the OD600 measurements recorded after 24 hours incubation. The growth profiles for the respective strains and cultures represent mean ± SD values obtain from two rounds of cultivation, each in technical triplicate (n = 6).
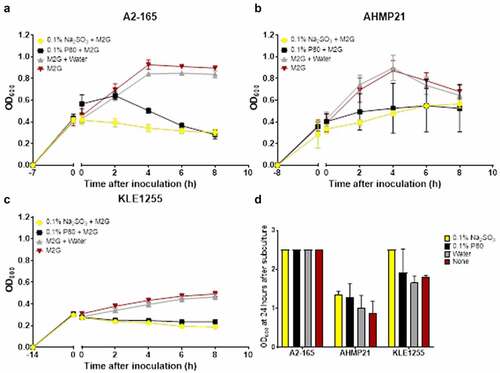
Figure 3. Saccharin and carboxymethylcellulose exert differential effects on Proteus mirabilis ProJ1 growth in microaerobic and aerobic conditions. Compared to their growth with the basal medium, microaerobic growth (doubling time, hours) appeared unaffected by any of the food additives tested (panel A). However, its aerobic growth was significantly increased (i.e., reduced doubling time) by the addition of carboxymethylcellulose, but decreased (i.e., increased doubling time) by the addition of saccharin. These data are presented as box plots showing mean ± SD, from no less than two rounds of cultivation, each in technical triplicate (n = 6), with the doubling times calculated and statistical comparisons made as described in the Methods (ns: not significant; ****P ≤ .0001).
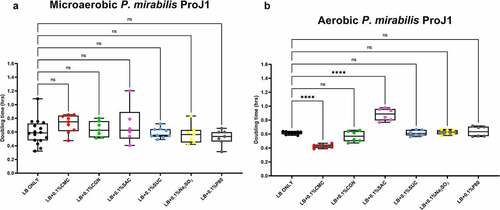
Figure 4. Sodium sulfite, polysorbate 80, saccharin, carrageenan, and carboxymethylcellulose exert differential effects on Morganella morganii growth in microaerobic and aerobic conditions. Compared to their growth with basal medium, the microaerobic growth of M. morganii strains CD10-6 (panel A) and CD10-12 (panel C) was negatively affected (i.e., increased doubling time) by the addition of either saccharin (P ≤ .05) or sodium sulfite (P ≤ .001). In contrast, the aerobic growth of both M. morganii strains tested was stimulated (i.e., reduced doubling times) by the additions of polysorbate 80 (P ≤ .01, panels B and D) and by sodium sulfite for strain CD10-6 (P ≤ .0001, panel B). Carrageenan significantly inhibited the aerobic growth of strain CD10-6, and both carrageenan and carboxymethylcelluose significantly inhibited the aerobic growth of strain CD10-12 (panels B and D). These data are presented as box plots showing mean ± SD, from no less than two rounds of cultivation, each in technical triplicate (n = 6), with the doubling times calculated and statistical comparisons made as described in the Methods.
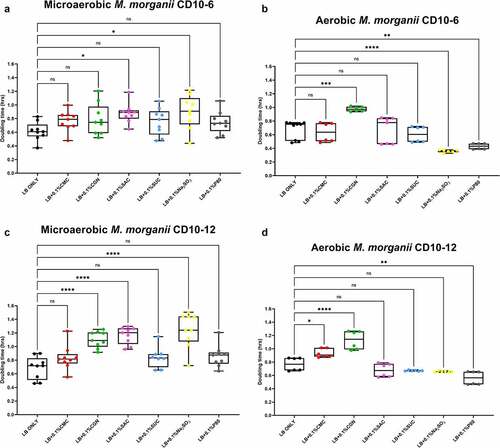
Figure 5. Sodium sulfite, saccharin, and carrageenan exert differential effects on Escherichia fergusonii growth in microaerobic and aerobic conditions. Compared to their growth with the basal medium, the microaerobic growth of E. fergusonii strains CD10-5 and CD10-10 was negatively affected (i.e., increased doubling time) by the addition of sodium sulfite (P ≤ .001, panels A and C) whereas there were no measurable effects on their aerobic growth by this additive (P > .05, panels B and D). Carrageenan inhibited the growth (i.e., increased doubling times) of strain CD10-10 under microaerobic conditions (panel C) and for both strains under aerobic conditions (panels B and D). Saccharin tended and significantly inhibited the growth of strain CD10-5 during microaerobic and aerobic growth, respectively (panels A and B) and significantly inhibited the growth of strain CD10-10 under microaerobic conditions (panel C). Interestingly, polysorbate 80 appeared to have no measurable impacts on the microaerobic or aerobic growth of both strains, and carboxymethylcellulose appeared to stimulate the aerobic growth of strain CD10-10 (panel C). These data are presented as box plots showing mean ± SD, from no less than two rounds of cultivation, each in technical triplicate (n = 6), with the doubling times calculated and statistical comparisons made as described in the Methods.
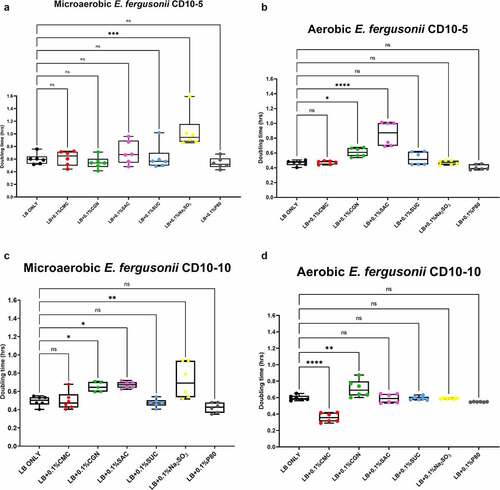
Figure 6. Sodium sulfite, saccharin, and carrageenan exert differential effects on Klebsiella pneumoniae growth in microaerobic and aerobic conditions. Compared to their growth with the basal medium, the microaerobic growth of both strains CD33-7 and CD34-4 was inhibited (i.e., increased doubling time) by sodium sulfite and saccharin (panels A and C). Except for microaerobic cultures of strain CD34-4, carrageenan also significantly inhibited the growth of both strains. Polysorbate 80 had limited effects on the growth of both strains except for a stimulatory (i.e., reduced doubling time) effect on the growth of strain CD34-4 in aerobic conditions (panel D). These data are presented as box plots showing mean ± SD, from no less than two rounds of cultivation, each in technical triplicate (n = 6), with the doubling times calculated and statistical comparisons made as described in the Methods.
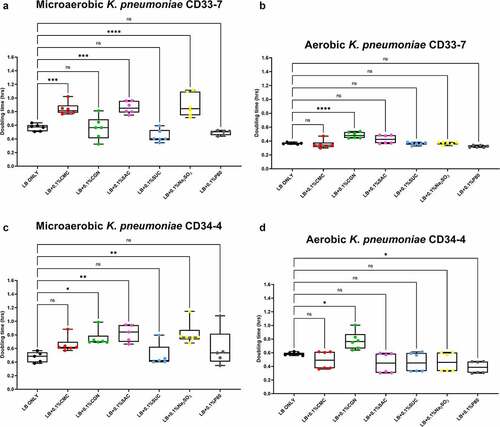
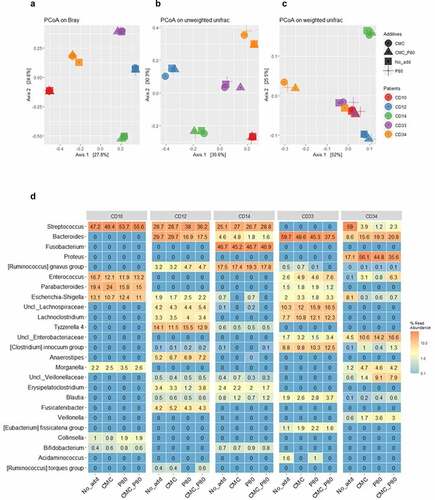
Figure 7. Principal Coordinates Analysis (PCoA) of the Bray Curtis dissimilarity (a), unweighted (b) and weighted (c) UniFrac metrics of the mucosa-associated microbiota from 5 post-operative CD patients, following their culture in habitat-simulating medium containing either no additions, or 0.1% final concentrations of either P80 and/or CMC. The legend for subject and medium composition are shown in panel A. The results suggest only the weighted UniFrac metric of the microbial consortia from subject CD34 undergoes dramatic changes in response to P80 and/or CMC. Panel D shows the heatmap displaying the top 25 predominant bacterial taxa (expressed as % read abundance) present in the microbial communities recovered from the 5 post-operative CD patients following growth with either basal medium (No_add), or the basal medium supplemented with 0.1% (final concentration) of either carboxymethylcellulose (CMC), polysorbate P80 (P80), or both food additives (CMC_P80). Note the subject consortium-specific effects from the food additives on members of Streptococcus and Bacteroides spp., and particularly for CD34, the effects from P80 on the increased relative abundances of Veillonella, unclassified Family_Veillonellaceae (Uncl._ Veillonellaceae), and Morganella spp. in cultures emended with P80 only. There is also an increased relative abundance of Proteus, unclassified Family_Enterobacteriaceae (Uncl._ Enterobacteriaceae), and Enterococcus spp. within cultures containing P80 and/or CMC, and reductions in the relative Escherichia-Shigella in response to these additives. Panel d reproduced with permission from Kang et al.Citation55