Figures & data
Figure 1. Synthetic approach to making anti-parasite effector genes. A synthetic approach to making an anti-parasite effector gene starts with a simple model of a gene (A) comprising two parts. The control region contains cis-acting DNA that regulates when during development, where in the vector insect, and how much of a product is made. Constitutive and regulated endogenous mosquito genes with sex-, stage- and tissue-specific expression profiles have been used for the control regions (Table ). The effector region is the expressed portion of the gene that kills or disables the parasite. This may result from a direct action such as a single-chain antibody that binds the parasite or toxin that kills it, or an indirect action that deprives the parasite of an essential host factor, blocks an important ligand or elevates a systemic immune response (Table ). (B) Control regions can be selected to deliver effector molecules to specific compartments (midgut, hemolymph [open circulatory system] and salivary glands) in which specific parasite stages are found.
![Figure 1. Synthetic approach to making anti-parasite effector genes. A synthetic approach to making an anti-parasite effector gene starts with a simple model of a gene (A) comprising two parts. The control region contains cis-acting DNA that regulates when during development, where in the vector insect, and how much of a product is made. Constitutive and regulated endogenous mosquito genes with sex-, stage- and tissue-specific expression profiles have been used for the control regions (Table 2). The effector region is the expressed portion of the gene that kills or disables the parasite. This may result from a direct action such as a single-chain antibody that binds the parasite or toxin that kills it, or an indirect action that deprives the parasite of an essential host factor, blocks an important ligand or elevates a systemic immune response (Table 1). (B) Control regions can be selected to deliver effector molecules to specific compartments (midgut, hemolymph [open circulatory system] and salivary glands) in which specific parasite stages are found.](/cms/asset/a8c7d333-746a-4691-b5ee-e31fa8831a00/ypgh_a_1427192_f0001_oc.gif)
Table 1. Antimalarial effector genes and targets.
Table 2. Anopheline mosquito cis-acting DNA elements for expressing effector molecules.
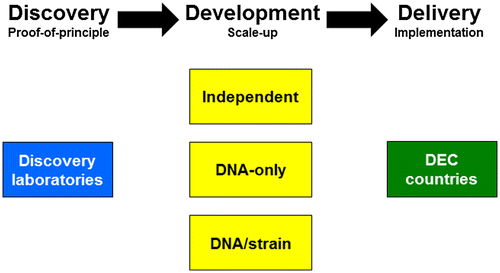
Figure 2. Genotypic and phenotypic outcomes of gene-drive systems.
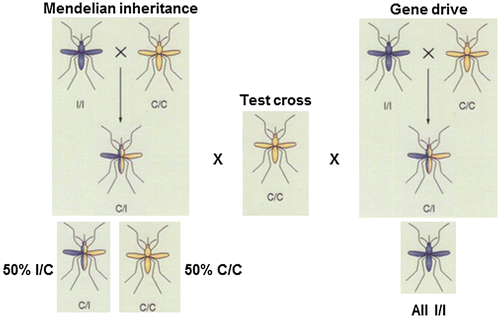
Figure 3. Pathways for development of population modification technologies.