Figures & data
Figure 1. Biofilm formation in different Pi concentration media. Selected isolates (MGP16, MGP12, and MGP45) were grown in static conditions for 48 h in M63 medium modified with the indicated Pi concentrations. Biofilm formation was quantified by cristal violet technique. Data represent the mean ± SD of at least 3 independent experiments. Different letters indicate significant differences according to Tukey's test with a p-value ≥ 0.05.
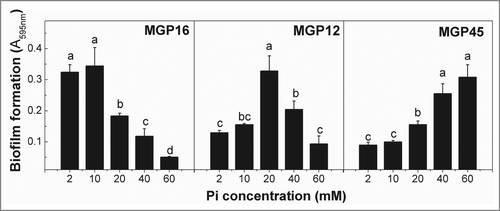
Table 1. Pi concentrations established to each biofilm formation conditions
Figure 2. Microscopy analysis of E. coli isolates. Biofilm formation of the indicated E. coli isolates grown in static conditions for 48 h in M63 medium in BF+ and BF– conditions was analyzed (A) by CLSM and represented by 3-dimensional reconstruction of biofilm image or (B) by SEM microscopy, magnification: 5,000x. Inserts i and ii, 30,000x; insert iii, 80,000x; insert iv, 20,000x. Data are representative from 2 independent experiments.
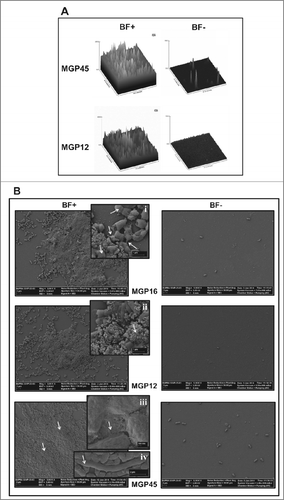
Figure 3. Biofilm associated phenotypes of E. coli isolates. (A) Aliquots of bacteria in an OD560nm = 0 .1 were spotted on solid M63 plates supplemented with different Pi concentrations (BF+ or BF- conditions). Plates containing Congo red dye and brilliant blue were incubated at 30°C for 48 h. The presence of curli fiber was observed as purple or red colonies. The colorless colony by csgA mutant, defective in an essential curli component, was used as negative control. (B) Bacteria were grown statically on glass covers in M63 liquid medium supplemented with the indicated Pi concentrations. After 48 h, attached cells were treated with Calcofluor White. The presence of cellulose was observed in an optical fluorescence microscope with 100x magnification. (C) Bacteria were grown for 48 h on semisolid 0.3% agar M63 plates with the indicated Pi concentrations. Motility was expressed as colony diameter. In all cases, results represent at least 4 independent experiments performed in duplicate.
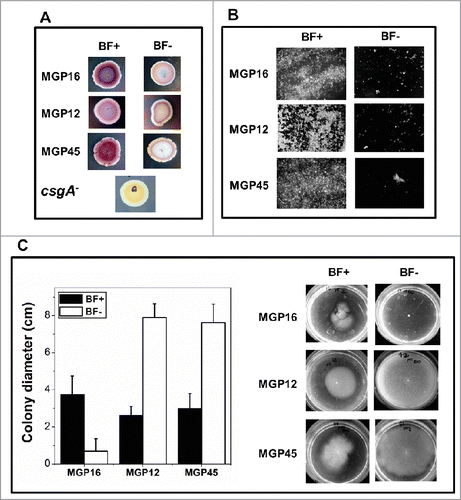
Figure 4. Relative expression of virulence factors related to biofilm formation. Expression of fimB, iroN and hlyA genes of the selected isolates was determined by q-PCR. Values are depicted as x-fold of up- or down-regulation of BF+ conditions compared to BF- conditions. Data represent the mean ± SD of at least 2 independent experiments performed in triplicate.
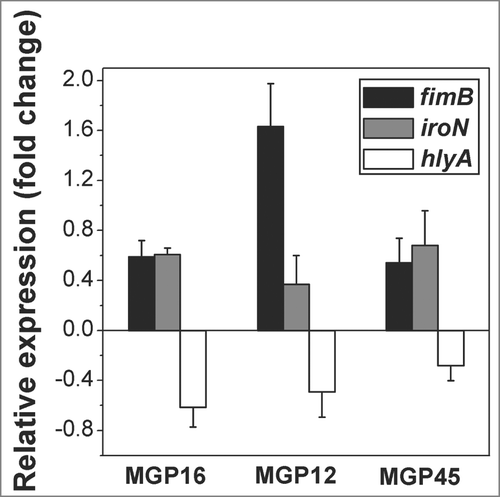
Figure 5. Antibiotic resistance in static conditions. ON cultures of the selected isolates were diluted to an OD560nm=0 .1 and incubated in multiwell plates in M63 medium containing different Pi concentrations and the indicated nalidixic acid concentrations. Plates were incubated at 30°C for 48 h and biofilm formation was assessed by cristal violet staining. Results represent the mean ± SD of 3 different experiments.
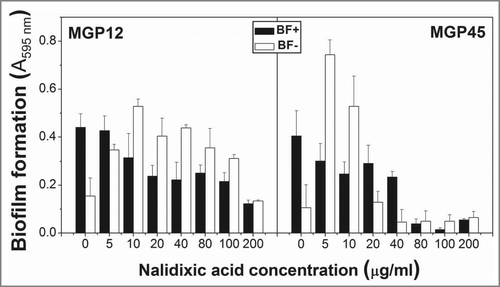
Figure 6. Intracellular polyP levels and biofilm formation in parental and mutants strains. (A) Cells of the indicated strains were grown for 48 h in static M63 medium modified with the Pi concentrations corresponding to BF+ (black line) and BF- (gray line) conditions. At the indicated time of growth, polyP was quantified using DAPI fluorescence, as described in Methods. DAPI emission was undetectable in cell free controls. (B) Indicated strains were grown in static conditions for 48 h in M63 medium modified with the indicated Pi concentrations corresponding to BF+ conditions (black columns) and to BF- conditions (white columns). Biofilm formation was quantified by cristal violet technique. Data are expressed as average ± SD of 4 independent experiments.
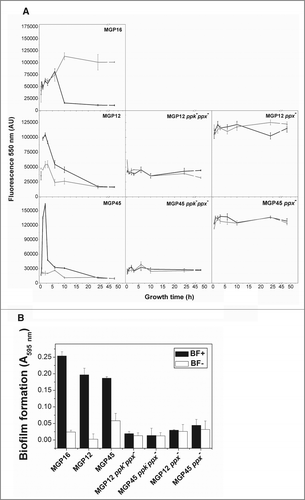
Table 2 Primers used for detection and expression of virulence factor genes
