Figures & data
Figure 1. Survival curves of G. mellonella infected with P. brasiliensis (A) and P. lutzii (B) at different concentrations; PBS-infected larvae were used as controls and statistical significance (p < 0.05) is relative to the PBS control.
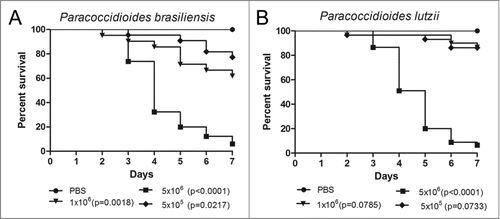
Figure 2. Histology of G. mellonella stained with PAS. Uninfected larvae (A, B); larva infected with P. brasiliensis after 1 hour (C) and after 4 days (E); larva infected with P. lutzii after 1 hour (D) and after 4 days (F). Amplification 1000x. Arrows indicates P. brasiliensis or P. lutzii. Structures annotated: (a) cuticle; (b) adipose bodies; (f) fungal cells.
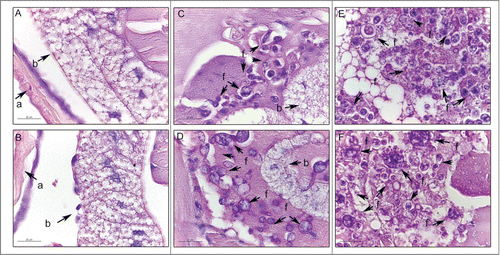
Figure 3. The cellular and humoral response to infection with P. brasiliensis after 3 days. Granuloma structure, amplification 400X (A); melanization and encapsulation process, amplification 1000x. (B). Similar structures were observed during P. lutzii infection. Structure annotated: (g) granuloma-like structure.
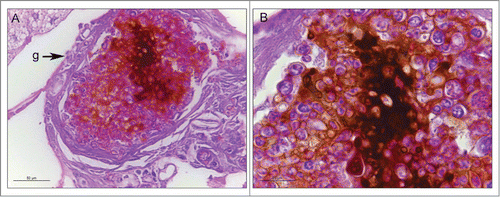
Figure 4. Hemocyte density, as obtained by microscopy (A) and flow cytometry (B), in G. mellonella larvae infected with P. brasiliensis (Pb18) and P. lutzii (Pl01) when assessed after 1 and 3 h. The asterisks indicate statistical significance (p < 0.05) relative to the PBS control.
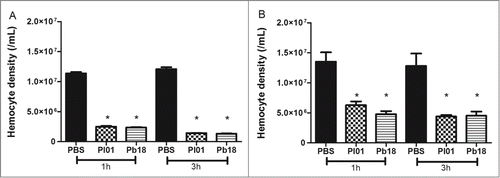
Figure 5. Linear regression and scatter plots for hemocyte counts (/mL) obtained by flow cytometry and microscopy (manually) in G. mellonella larvae injected with PBS, P. brasiliensis (Pb18 - 5×106 cells/larva) or P. lutzii (Pl01 - 5×106 cells/larva) that were assessed after 1 and 3 h.
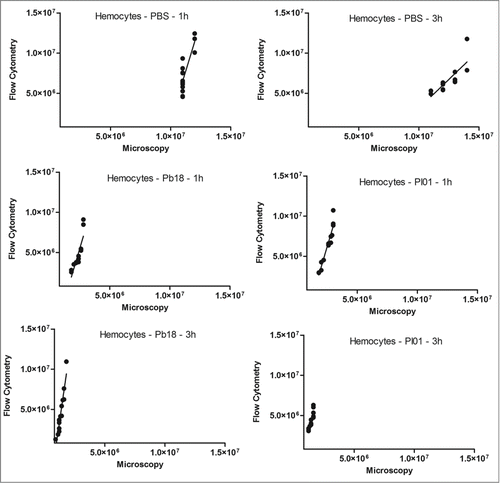
Figure 6. Hemocyte-fungal interaction obtained by flow cytometry after infection with P. brasiliensis (A) and P. lutzii (B). In gate P1, the hemocyte population is phalloidin positive with non-infected larvae; in gate P2, the hemocyte population is phalloidin-positive with infected larvae; and gate P3 holds the doubly stained population (hemocyte-stained phalloidin and fungal-stained CFDA-SE) that are considered hemocyte-fungal interactions that were obtained by flow cytometry after infection with P. brasiliensis (A) and P. lutzii (B). Differences in the hemocyte-fungal interaction between the fungal species (p < 0.05).
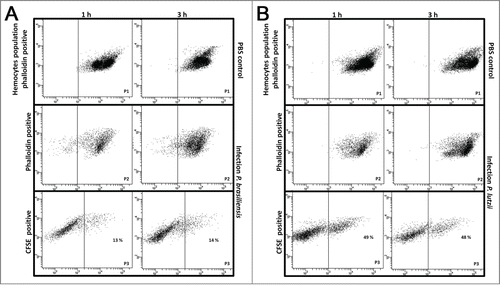
Figure 7. Phagocytosis of Paracoccidioides spp by hemocytic cells after 3 h of infection with 5×106 cells/larva from P. brasiliensis (A) and P. lutzii (B). The arrow indicates the phagocytosis.
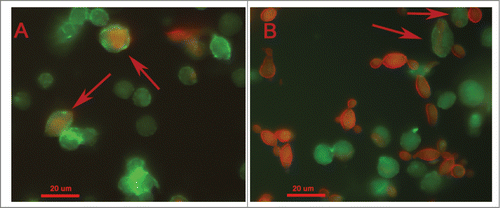
Figure 8. Relative gene expression of enolase, gp43, 14-3-3, triosephosphate isomerase and malate synthase in P. brasiliensis (black) and P. lutzii (white), (*) p < 0.05.
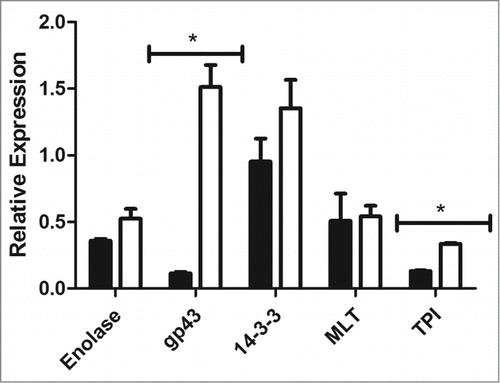
Figure 9. Western blot of G. mellonella infected with P. brasiliensis or P. lutzii. 1) molecular weight marker; 2) P. lutzii; 3) G. mellonella infected with P. lutzii; 4) P. brasiliensis; 5) G. mellonella infected with P. brasiliensis; 6) G. mellonella extract infection; and 7) purified gp43. The arrow indicates the gp43.

