Figures & data
Figure 1. APEC and NMEC strains exhibit distinct cell association and invasion patterns in RAW 264.7 macrophages. Cell association (A) and invasion (B) patterns of APEC and NMEC strains in RAW 264.7 macrophages were performed as described in the Materials and Methods. The OmpA− mutant of E44 (E91) was used as a negative control. Cell association and invasion experiments were performed at least 3 times in triplicate, and the values are presented as percent means ± S.D considering E44 invasion as 100%. Increase or decrease in cell association/invasion was statistically significant for some strains compared to E44, *p < 0.05 by Student's t test.
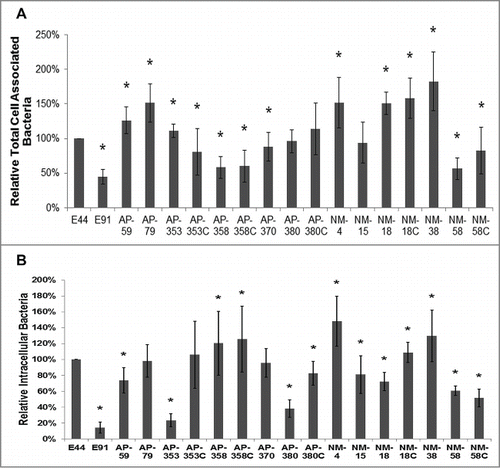
Table 1. The genotypes of all strains of E. coli used in this study
Figure 2. APEC and NMEC strains evade serum complement killing and survive inside bone marrow derived macrophages (BMDMs). Serum survival assays were performed with the APEC (AP) or NMEC (NM) strains as described in the Materials and Methods in the presence of normal and heat-inactivated serum, and percentage survival after 15 minutes exposure was compared to the corresponding values at 0 minutes for each individual strain. The decrease in survival with normal serum or increase in survival with heat-inactivated serum is statistically significant, *p < 0.05 by Student's t test. (A). Cell association (B) and invasion (C) patterns of the strains in BMDMs were also analyzed. The serum survival and cell association/assays were performed at least 3 times in triplicate, and the values are presented as percent means ± S.D considering E44 invasion as 100%. Increase or decrease in cell association/invasion was compared to E44, *p < 0.05 by Student's t test.
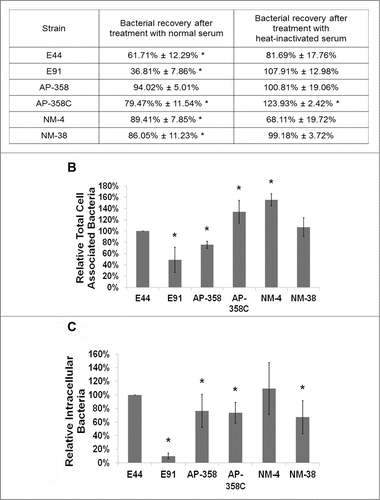
Figure 3. APEC and NMEC strains require CD64 for macrophage invasion, similar to E44. RAW 264.7 macrophages were infected with the different APEC (AP) and NMEC (NM) strains, and the infected macrophages were subjected to flow cytometry analysis to determine the surface expression of CD64 in response to infection (A). RAW 264.7 macrophages were transfected with siRNA to CD64, and the cells were allowed to recover for 24 h before performing invasion assays with the strains (B). The effect of CD64 silencing by the siRNA was concurrently verified by flow cytometry (C). The invasion assays were performed at least 3 times in triplicate, and the values are presented as percent means ± S.D considering the bacterial invasion of untransfected cells as 100%. Decrease in the invasion of the strains in the presence of siRNA was statistically significant compared to untransfected cells, *p < 0.05 by Student's t-test.
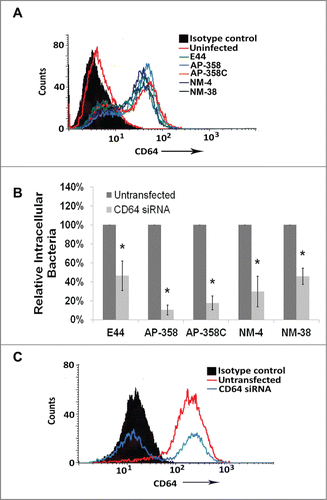
Figure 4. APEC and NMEC strains manipulate Ecgp96 for brain endothelial cell invasion. Cell association (A) and invasion (B) patterns of APEC (AP) and NMEC (NM) strains in HCMEC/D3 were performed. APEC and NMEC infected HCMEC/D3 were subjected to flow cytometry to examine the surface expression of Ecgp96 in response to infection (C). HCMEC/D3 were transfected with siRNA to Ecgp96, and the cells were allowed to recover for 24 h before performing invasion assays with the strains. The percent reduction in invasion of each strain in the presence of siRNA was calculated relative to their invasion taken as 100% in the absence of siRNA (D). Efficient Ecgp96 silencing was confirmed by flow cytometry (E). The cell association/invasion experiments were performed at least 3 times in triplicate and the bacteria bound or invaded are presented as percent means ± S.D taking the total cell association or invasion of E44 as 100%. Increase or decrease in cell association/invasion of the strains was statistically significant compared to untransfected HCMEC/D3, *p < 0.05 by Student's t-test.
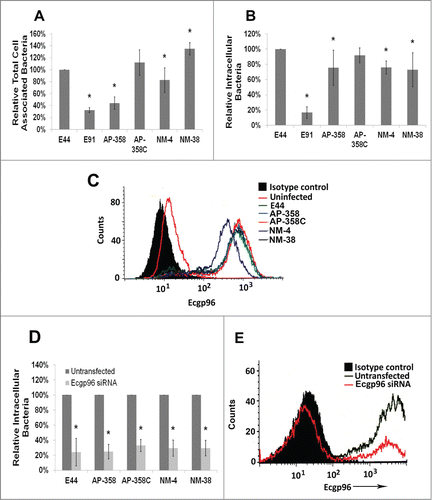
Figure 5. APEC (AP) strains cause meningitis similar to that of NMEC (NM) strains. Three-day-old mice were infected with 100 CFU of each of the strains via the intraperitoneal route and the bacterial load in blood (A) and brain (B) were enumerated. Excluding E91, statistical analysis by one-way ANOVA indicates no significant difference in blood and brain bacterial loads among the APEC and NMEC strains when compared to E44. No colonies were recovered from E91 infected pups. The summary of the animal experiments are tabulated (C). H&E staining of the brain sections was performed to assess the pathological changes (D). Arrows indicate the presence or loss of meninges.
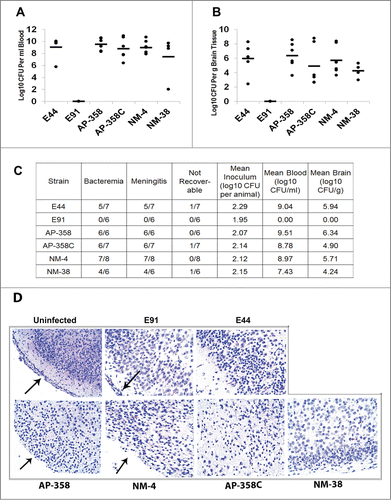
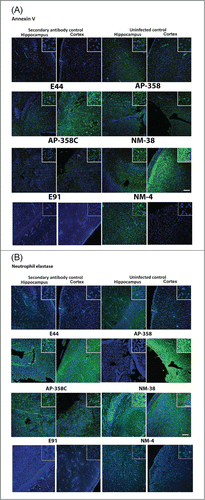
Figure 6. Patterns of neuronal apoptosis and neutrophil infiltration in the brains of infected newborn mice were distinct for each APEC (AP) or NMEC (NM) strain. Sections adjacent to the ones used for H&E staining, were subjected to immunofluorescence staining with Annexin V (for apoptosis) (A) and neutrophil elastase (for neutrophil infiltration) (B) in the cortex and hippocampus regions. Insets show magnified areas in the sections. Scale bar = 100 µm.