Figures & data
Figure 1. CpAls7 disruption strategy based on SAT1 flipper cassette (A). Upstream and downstream homology sequences from C. parapsilosis reference strain ATCC 22019 were amplified and inserted at the ApaI/XhoI and SacII/SacI sites surrounding the SAT1 flipper cassette. The disruption cassette integrated in the CpALS7 allele by homologous recombination. (B). Southern blot hybridization analysis of genomic DNA isolated from the mutants collection and digested with BsmI restriction enzyme. The probe used to verify the correct construction of the mutant collection was amplified by PCR from the downstream homology fragment (schematized by the black bar). M: Roche Dig Labeled Marker VII; 1: wild type (WT); 2: heterozygous with cassette (HC); 3: heterozygous (H); 4: null mutant with cassette (KOC); 5: null mutant (KO); 6: disruption cassette. The expected sizes were 6.4 Kb, 1.9 Kb, 2.9 Kb, and 5.3 Kb for the wild type allele, the allele with the integrated cassette, the deleted allele and the cassette, respectively. (C). The entire coding sequence of CpALS7 was amplified and cloned at the 5′ end of the SAT1 flipper cassette. The reintegration cassette integrated in one of the 2 null mutant alleles by homologous recombination. Primers OM4UPF2 and OM4DWR1 (Table S2) were used to verify the presence of the entire copy of CpALS7 integrated in the correct locus in reconstituted strain (R). Expected fragment lengths: 4.2 Kb for WT and R, 852 bp for KO strains. M: 1Kb DNA ladder (Invitrogen), 1: WT; 2: KO; 3: R; CN: negative control. (D). WT and mutant strains (HC, H, KOC, KO, reconstituted strain with cassette (RC), R) grown for 48 h at 30°C on YPD or nourseothricin (NTC) supplemented YPD plates.
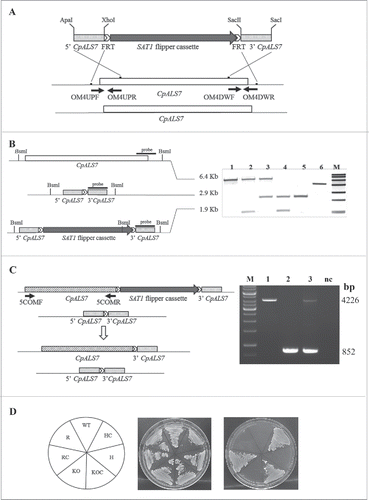
Figure 2. Phenotypic analysis of C. parapsilosis strains. (A). Growth curve of wild type strain (WT, ATCC22019), CpALS7 heterozygous (H), null (KO) and reconstituted (R) strains in YPD medium at 37°C. (B). Susceptibility to cell wall perturbing agents of the C. parapsilosis strains was evaluated by spot assay, on YPD agar supplemented with the following compounds: fluconazole (0.5 mg/l), congo red (1 mg/l), caffeine (5 mM); calcofluor white (20 mg/l). Approximately 1 × 106 cells and 10 fold dilutions were spotted on different media. Plates were incubated at 30°C (Fig. S1) or 37°C for 48 h, and visually inspected. Experiments were performed in duplicate, with similar results. (C). Production of pseudohyphae by CpALS7 mutant strains. C. albicans SC5314 was also included as positive control. Morphogenesis was induced in YPD broth in presence of 10% FBS. Following 24 h of incubation at 37°C, 10 μl of each culture was directly observed with an optical microscope at 400 × magnification. (D). The ability to produce filaments was also visualized on colony borders grown on spider agar. Photographs were taken following 7-day incubation at 37°C. C. albicans SC5314 represented a positive control for morphogenesis.
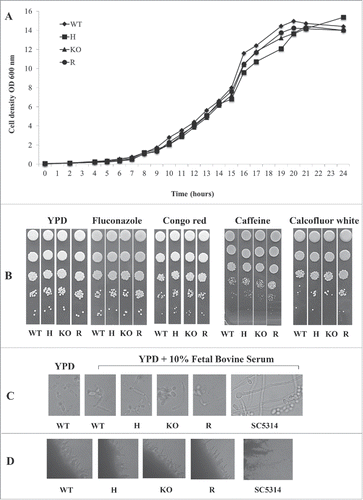
Figure 3. Basal transcriptional profiles of all 5 putative ALS like genes in C. parapsilosis wild type (WT), CpALS7 heterozygous (H), null (KO) and reconstituted (R) mutant strains grown in YPD medium to late exponential-phase. (A). Relative expression heat map of ALS-like genes normalized on actin expression levels. (B-F). Relative expression of each gene in mutant strains normalized on actin and wild type transcripts. At least 3 biological replicates were analyzed. Error bars represent standard error of means of 3 independent experiments.
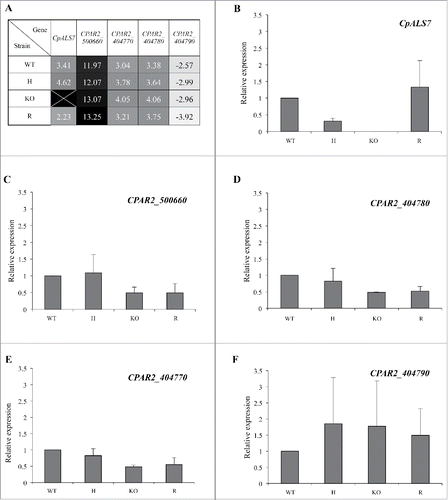
Figure 4. The adhesion ability of C. parapsilosis wild type (WT), heterozygous (H), null (KO) and reconstituted (R) mutant strains was tested on human buccal epithelial cells obtained from a healthy donor not colonized with Candida spp. Bars represent adhesion index mean ± standard error of mean. At least 3 biological replicates were used. ***P < 0.001. Micrographs below bars show representative Gram-stained C. parapsilosis blastoconidia from each of the strains adhered to a buccal cell observed at a magnification of 1000 ×. Scale bar denotes 10 μm.
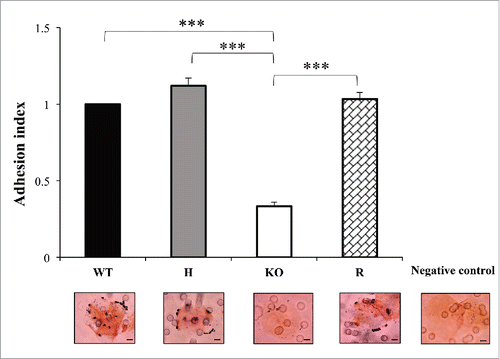
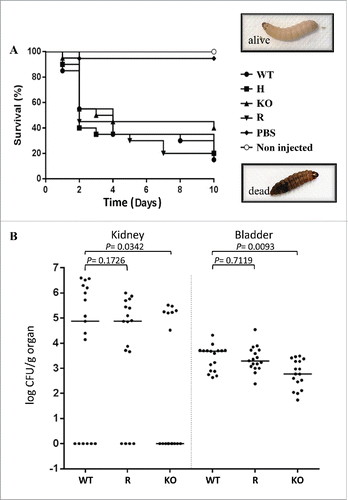
Figure 5. Effect of deletion of CpALS7 on C. parapsilosis pathogenicity. (A). Intra-hemocelic infection of Galleria mellonella larvae with CpALS7 wild type (WT) and CpALS7 mutant strains (heterozygous, H; null, KO; and reconstituted, R). Survival curves of G. mellonella infected with CpALS7 wild type and lineage b mutant strains (20 larvae per group) with 8×105 CFUs per larva. Photographs represent typical pigmentation of alive and dead larvae. (B). Murine model of urinary tract infection. Groups of 17 BALB/c mice were transurethrally challenged with approximately 1 × 108 C. parapsilosis cells for each of the indicated strains. Data are expressed as the log10 colony-forming units (CFUs)/g of yeast cells recovered from kidney and urinary bladder homogenates 4 days after the challenge. The log10 CFUs from both kidneys were combined and averaged. A value of 0 was assigned to uninfected organs. Horizontal bars represent median. Log10 counts were compared for statistical significance by non-parametric Wilcoxon rank sum tests. A P value < 0.05 was considered to be statistically significant.