Figures & data
Figure 1. Senescence and the DNA damage response. Different stimuli can trigger replicative senescence or stress-induced premature senescence through activation of the DNA damage response. Telomere shortening at each DNA replication or alteration of the shelterin complex (e.g., depletion of TRF2, Telomeric repeat-binding factor 2) leads to the unmasking of chromosomes extremities. Oncogene activation leads to deregulated proliferation, promoting replication errors. Oxidative stresses and various other agents can trigger extensive non-telomeric DNA damage.
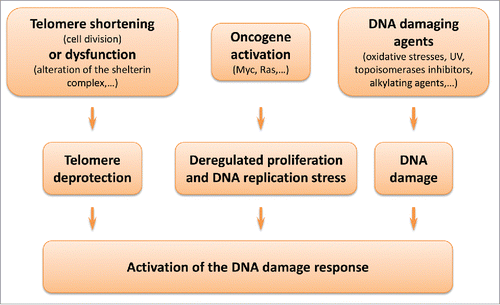
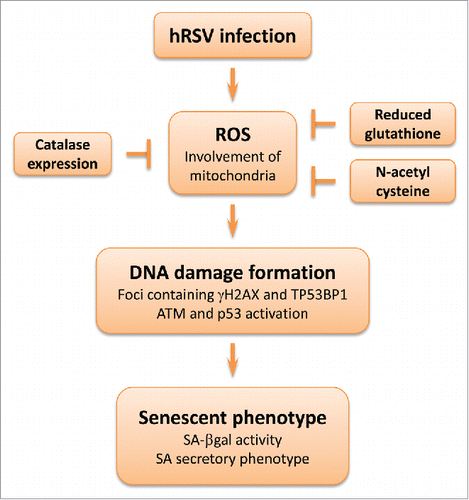
Figure 2. hRSV-induced premature senescence. Cell infection by hRSV leads to generation of ROS, including superoxide anions derived from mitochondria. The oxidative stress is sufficient to trigger DNA damage as noted by the detection of nuclear foci containing TP53BP1 (tumor protein p53 binding protein 1) and γH2AX (in presence of DNA damage, histone variant H2AX is phosphorylated on serine 139 by the ATM kinase or other kinases). Ultimately, cells develop the senescent phenotype. Antioxidants can protect against this process: N-acetyl cysteine, catalase expression or reduced glutathione decrease the oxidative stress as well as DNA damage formation.