Figures & data
Figure 1. FipB migrated as high molecular weight complexes that were sensitive to reduction. FipB protein was purified from strains expressing His-tagged wild-type FipB, FipB-CXXA, or FipB P286T, using a metal affinity column. FipB was eluted from the column and run on separate SDS-PAGEs using sample buffer without (−DTT, Panel A) or with DTT (+ DTT, Panel B), and then transferred to a nylon membrane for Western blotting. FipB complexed with co-purified substrates were visualized with anti-FipB antibody.
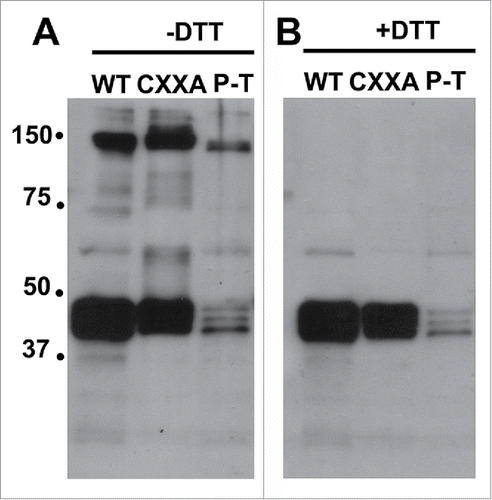
Figure 2. Accessibility of free sulfhydryls in IglB and IglC in wild-type and ΔfipB mutant bacteria. Panel A) Total bacterial lysates were labeled with AMS, a reagent that reacts with free sulfhydryls and adds 500 Da. Some samples were first treated with TCEP, to reduce existing disulfide bonds. Samples were separated on 4–15% SDS gel before transfer to PVDF membranes for immunoblots. Proteins were visualized with anti-IglC and IglB monoclonal antibodies. The same blot was stripped and then rehybridized with anti-FupA antibody. Blots are representative of at least three blots. Panels B& C) Blots were scanned by densitometry, and the amount of IglB (Panel B) or IglC (Panel C) was compared to the loading control FupA.
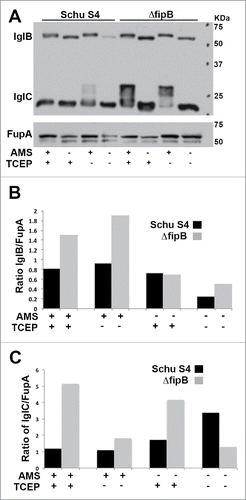
Table 1. List of putative FipB substrates
Figure 3. FipB resolved higher MW complexes of IglC and prevented higher MW complexes of FopA. Panel A) His-IglC was incubated with AMS and increasing concentrations of DTT in the presence or absence of His-FipB. Panel B) Western blot of total cell lysates of wild-type, ΔfopA, and ΔfipB strains. The same blot was incubated with anti-FopA antibody, then stripped and incubated with anti-FipB, and then stripped again and incubated with anti-FupA antibody.
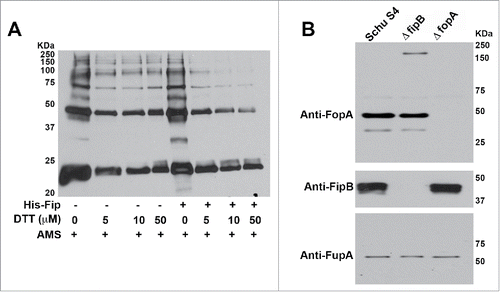
Figure 4. Detection of nonsecreted T6SS component and outer membrane constituents in the supernatants of KCl-grown bacteria. IglB, a nonsecreted T6SS component, outer membrane protein FopA, and LPS were detected in culture supernatants of KCl-grown bacteria. Bacterial cultures were grown overnight with or without 2.5 % KCl, and adjusted to the same OD595. Whole cell lysates and culture supernatants were prepared as described in material and methods. The Western blot was incubated with anti-IglB and IglC antibodies, then stripped and incubated with an anti-FopA antibody. The same blot was stripped again and incubated with an anti-LPS antibody. Statistical significance was measured using an ANOVA and Dunn's multiple comparison tests (* p value <0 .05).
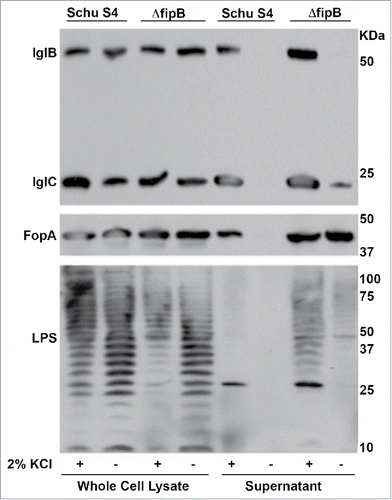
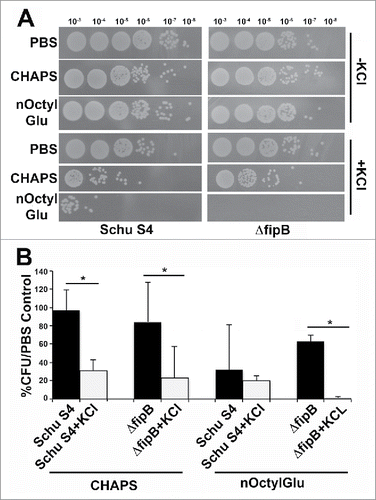
Figure 5. Growth in 2.5% KCl increases sensitivity to detergents. Schu S4 or ΔfipB strains were grown overnight in TSB/c with or without 2.5% of KCl. Cultures were adjusted to an OD595 of one, then incubated with 0.25% CHAPS or n-Octyl glucoside (nOctylGlu) for 90 min at RT. Serial dilutions, (by a factor of ten), were spotted on MHA/c plates and incubated at 37 ˚C for two d (Panel A). Quantitation of the detergent sensitivity was determined by comparing the number of recovered CFUs compared to PBS-treated controls from at least three independent experiments (Panel B). Statistical significance was measured using an ANOVA and Dunn's multiple comparison tests (* p value <0 .05).