Figures & data
Figure 1. HCV-mediated regulation of hepcidin gene expression in vitro. A. qRT-PCR analysis of HAMP mRNA levels in (A1) HCV JFH-1- and (A2) mock-infected Huh7.5 cells. B. ELISA measurements of secreted hepcidin peptide in supernatants isolated from (B1) HCV-JFH-1- and (B2) mock-infected Huh7.5 cell cultures. Fold- changes in the infected cultures are presented as ratios of the mock-infected control (black bar). HCV NS3 mRNA expression (continuous line) was used to monitor HCV infection.
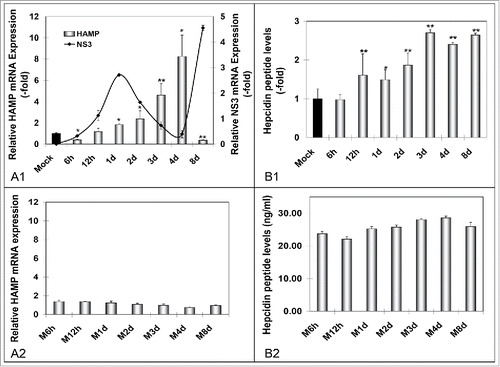
Figure 2. HCV modulates the expression of major iron homeostasis proteins. Representative Western blot analysis of expressed FPN, ferritin and HCV core in whole cell extracts from HCV JFH-1-infected hepatoma cells and respective molecular weights. β-actin was used as a loading control. (M: mock-infected control).
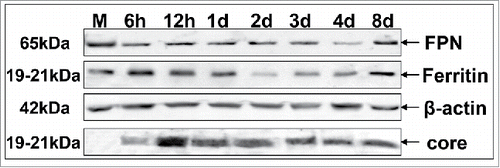
Figure 3. Hepcidin overexpression modulates HCV propagation. A. Validation of the hepcidin-overexpressing hepatoma cell line H21 by measurement of HAMP mRNA levels with qRT-PCR (A1), secreted hepcidin peptide levels with ELISA (A2), as compared to the “empty vector” cells (V24, black bar). A3. Immunoprecipitation of hepcidin peptide secreted by H21 cells with a monoclonal anti-hepcidin antibody. B. Electroporation of H21 cells with defective (B1) or wt (B2) HCV replicon RNA and RLA determination at various time points p.t. demonstrates viral translation and replication efficiency under conditions of overexpressed hepcidin. (M: marker).
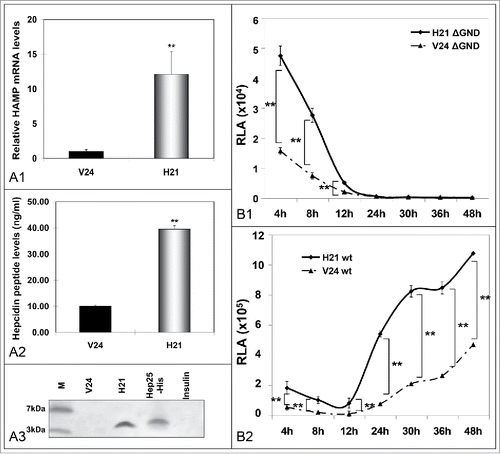
Figure 4. Hepcidin expression in HCV-infected hepatoma – macrophage co-cultures. A. Determination of HAMP mRNA from both cell lines following (A1) HCV-JFH-1 infection or (A2) mock infection. B. Measurement of secreted peptide levels from pooled supernatants in (B1) HCV-JFH-1- or (B2) mock-infected co-cultures at the given time points. NS3 mRNA expression in hepatoma cells was used to monitor HCV infection.
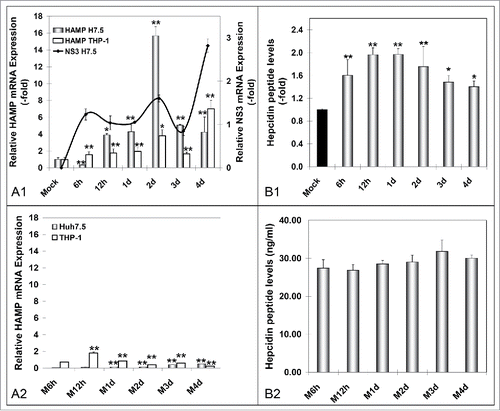
Figure 5. Attenuation of ferritin expression in HCV JFH-1-infected hepatoma cells in the presence of macrophages. Representative immunoblotting of whole cell extracts from infected co-cultures with antibodies against FPN, ferritin and β-actin (loading control). (M: mock-infected control).
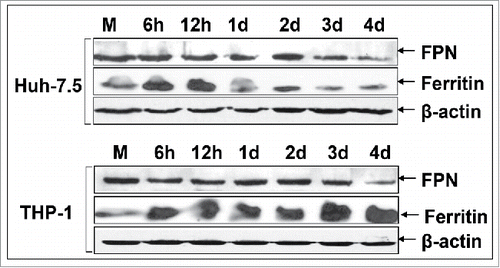
Figure 6. Macrophages co-cultured with HCV JFH-1-infected hepatoma cells support low levels of viral replication. HCV NS3 mRNA levels measured by qRT-PCR (A1) and HCV NS3 protein expression determined by Western blotting (A2) in co-cultured THP-1 macrophages. β-actin was used as a loading control. (M: mock-infected control).
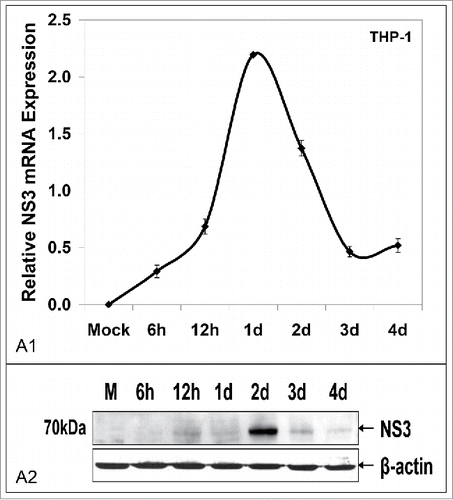
Figure 7. Iron loading of THP-1 macrophages enhances HCV replication in infected cells and promotes HCV infection in naïve cells. A. HCV NS3 mRNA (A1) and protein (A2) levels measured in infected hepatoma cells co-cultured with macrophages. B. Ferritin immunoblots in whole cell extracts from (A). C. HCV NS3 mRNA levels from THP-1 cells isolated from co-cultures of (A). D. HCV NS3 mRNA levels from naïve Huh7.5 cells co-cultured with infected THP-1 macrophages from (A).
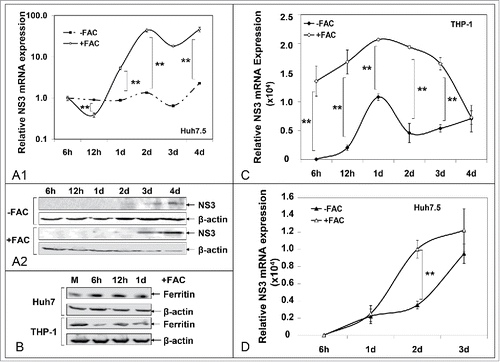
Figure 8. Differential regulation of secreted hepcidin peptide in vivo. ELISA measurements of hepcidin levels in sera of acute and chronic HCV patients.
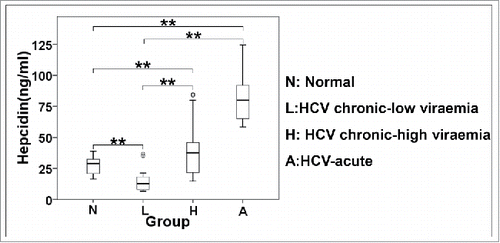
Table 1. Clinical and biochemical characteristics of enrolled subjects.
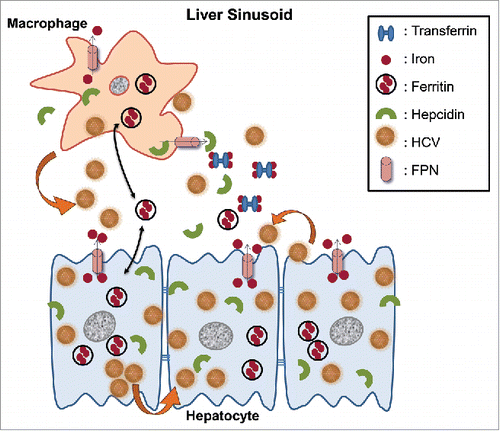
Figure 9. Schematic representation of putative interactions between HCV and the iron homeostasis network in hepatoma-macrophage co-cultures. We used an indirect hepatoma-macrophage co-culture system to simulate the co-existence of Kupffer cells and hepatocytes within the liver sinusoid. Upon viral egress, HCV particles infect a neighboring hepatocyte in a cell-free manner through specific plasma membrane receptors or in a direct cell-to-cell route via gap junction proteins (depicted by thick arrows). Iron is stored intracellularly in ferritin, leaves the cell through iron exporter ferroportin and circulates in blood bound to transferrin, or secreted ferritin. Hepcidin is produced by both hepatocytes and macrophages and regulates iron homeostasis via degradation of ferroportin, thus keeping iron within the cell. In our model, HCV mediated hepcidin up-regulation in hepatoma cells and accompanying macrophages, resulting in reduced ferroportin, elevated ferritin and iron levels intracellularly very early in infection. Later, a ferritin “flow” was observed from hepatoma cells toward macrophages (double pointed arrows). Hepcidin overexpression enhanced HCV translation and replication, and accompanied low levels of viral replication within macrophages. The ferritin “flow” could be reversed following macrophagic iron loading, which promoted enhanced viral replication in both cellular populations, as well as infection of naïve hepatoma cells through particle shedding (showed by a thick arrow).