Figures & data
Figure 1. Effect of Saps on neutrophil migration and on MIP-2 production. Human fluorescent neutrophils were incubated for 2 h to the upper compartment of transwell filters containing Medium, IL-8 (100 ng/ml), Sap2, tSap2 or Sap6 (all 0.5 µg/ml) in the presence or absence of Pepstatin A (1 µg/ml) (A, left) or vaginal epithelium, stimulated for 24 h in the presence or absence (Medium) of IL-8 (100 ng/ml), Sap2, tSap2 or Sap6 (all 0.5 µg/ml), pretreated or not with Pepstatin A (1 µg/ml) (A, right), in the lower compartment. The number of migrating neutrophils into the lower compartment was measured by using fluorescence signal. Data are expressed as fluorescence intensity of migrated neutrophils (triplicates samples of 5 different experiments, mean ± SEM). Human MIP-2 was assessed on cultures supernatants of vaginal epithelium stimulated for 24 h in the presence or absence (Medium) of IL-8 (100 ng/ml), C. albicans (CA-6) (1 × 107/ml), LPS (10 µg/ml) plus ATP (5 mM), Sap2 or Sap6 (both 0.5 µg/ml), pretreated or not with Pepstatin A (1 µg/ml), by specific Elisa assay (triplicates samples of 5 different experiments, mean ± SEM) (B). Human MIP-2 was also assessed on cultures supernatants of vaginal epithelium unstimulated (Medium) or stimulated for 24 h with C. albicans (CA-6) (1 × 107/ml), Sap2 or Sap6 (both 0.5µg/ml) in the presence or absence of Anakinra (10 µM) by specific Elisa assay (triplicates samples of 3 different experiments, mean ± SEM) (C). *, p < 0.05 IL-8, CA-6, LPS plus ATP or Saps treated vs Medium treated. #, p < 0.05 Saps + Pepstatin A treated vs Saps treated. # #, p < 0.05 CA-6 or Saps + Anakinra treated vs CA-6 or Saps treated.
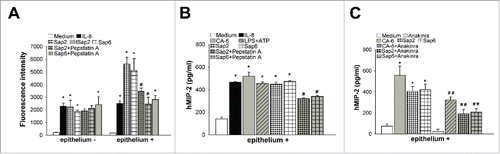
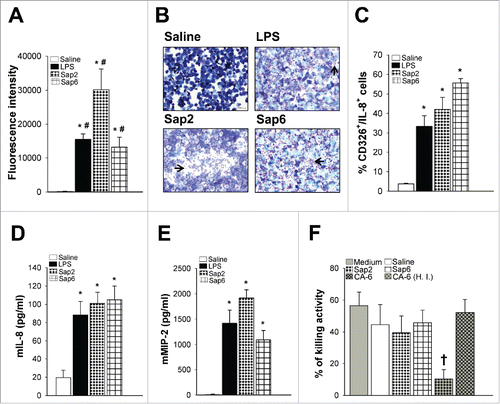
Figure 2. Neutrophil influx and activity during vaginal candidiasis. Human fluorescent neutrophils were incubated for 2 h to the upper compartment of transwell filters containing, in the lower compartment, vaginal washes of mice injected for 24 h with Saline, LPS (50 µg/10 µl/mouse), Sap2 or Sap6 (both 0.5 µg/10 µl/mouse). The number of migrating neutrophils into the lower compartment was measured by using fluorescence signal. Data are expressed as fluorescence intensity of migrated neutrophils (triplicates samples of 3 different experiments, mean ± SEM) (A). Vaginal washes of mice injected as above described were centrifuged, cellular fraction was microscopically analyzed to evaluate neutrophil recruitment (arrow) (representative images of 3 separate experiments with similar results, magnification 10 x, Bar = 50 µm) (B) or the % of double positive CD326+/IL-8+ cells (triplicates samples of 3 different experiments, mean ± SEM) (C) and supernatants were collected and tested for mouse IL-8 (D) and mouse MIP-2 (E) production by specific Elisa assays (triplicates samples of 3 different experiments, mean ± SEM). Killing activity against C. albicans of human neutrophils mixed with Medium or vaginal washes of mice injected for 24 h with Saline, Sap2 or Sap6 (both 0.5 µg/10 µl/mouse), C. albicans (CA-6) (2 × 107/10µl/mouse) or H. I. vaginal washes of mice injected for 24 h with C. albicans (CA-6) (2 × 107/10µl/mouse) was shown (triplicates samples of 5 different experiments, mean ± SEM) (F). *, p < 0.05 LPS or Saps treated vs Saline treated mice. #, differences between LPS, Sap2 or Sap6 treated animals resulted not significant. †, p < 0.05 CA-6 treated vs Medium treated mice.