Figures & data
Figure 1. Bactericidal activity of different serum samples toward a panel of clinical ST258 isolates. 3–5 × 103 CFU of the indicated ST258 isolates were incubated at 37 °C in 75 % serum samples of different origin as indicated on the individual panels. Aliquots were plated after incubation for 1 h and 2 h respectively and the recovered CFUs were correlated with the inoculum. The detection limit was 0.1% of inoculum. NHS: normal human serum.
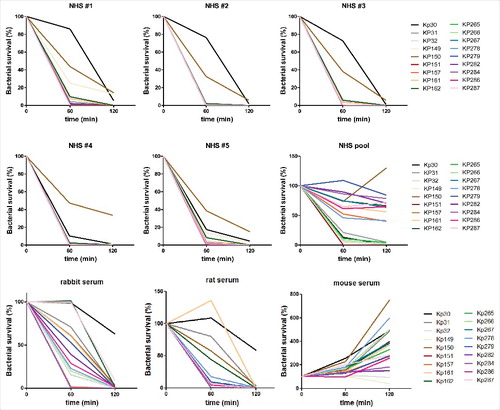
Table 1. Equilibrium and kinetic rate constants for the binding of mAb A1102 to the biotinylated D-galactan-III antigen, determined with either the Fab fragment or the IgG in Forte-Bio in PBS, pH 7.2, containing 1% BSA at 30 °C.
Figure 2. Endotoxin neutralization by a gal-III specific mAb in vitro. Endotoxin signaling through TLR4 was measured in a cell-based colorimetric assay in the presence of mAb A1102. (A) using purified LPS extracted from 3 isolates expressing gal-III (KP150, KP151, PCM-27). A1102 mAb (red curves), isotype matched control mAb (black curves), or polymyxin B (PMB, blue curves) were used at the indicated concentrations. (B) using shed LPS in supernatants of mid-log phase cultures of ST258 isolates as well as strain PCM-27. (C) Neutralizing potency of A1102 compared with its fragments as well as an aglycosylated (N297Q) version.
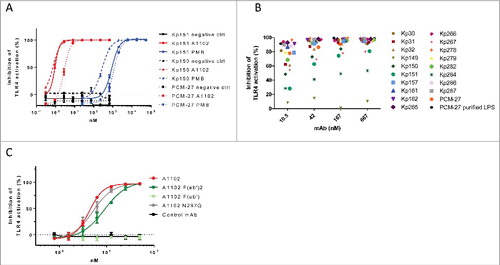
Figure 3. Protection in murine endotoxemia models. Protective efficacy of mAb A1102 was investigated in the GalN-sensitized murine model of endotoxemia. Groups of 5 mice were passively immunized prophylactically (i.p.) with serial dilutions of A1102 (100 µg to 0.78 µg/mouse doses) or an isotype-matched control antibody (100 µg/mouse). Mice were rendered susceptible to endotoxin by receiving an i.p. injection of GalN 24 h later and at the same time were challenged i.v. with either purified LPS, extracted from a representative ST258 strain, Kp151 (16 ng/mouse) (panel A), or with a minimal lethal dose (1.5–5 × 104 CFU/mouse) of ST258 strains (panel B). Survival was monitored daily for up to 10 d. Graphs represent the combined results from 2 independent experiments for each challenge, with a total group size of 10, except for mice receiving the 0.78 µg dose in live challenges (panel B), which was only included in one of the repeats (n = 5).
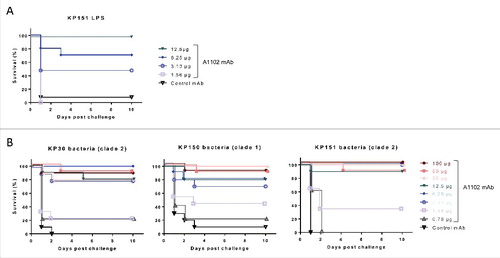
Figure 4. Protection in a rabbit model of bacteremia. Groups of 4 rabbits were immunized i.v. with the indicated doses of A1102 or an isotype-matched irrelevant control mAb. Next day, the animals were challenged i.v. with a lethal dose (5 × 109 CFU/kg) of ST258 strain Kp151. Infected animals were monitored every 3 h for the first day and then daily for 7 d. The graph represents combined data from 2 independent experiments (n = 8 per group in total) with similar outcome.
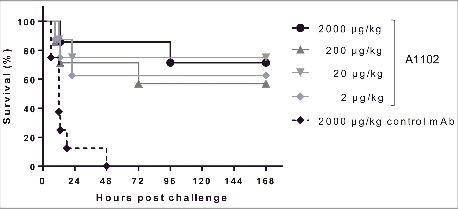
Figure 5. Antibody dependent bactericidal activity in human and rabbit sera. ST258 strains (A: Kp32 or B: Kp151) were incubated with 50 % adsorbed human (A) or rabbit (B) serum for up to 60 min in the presence of either A1102 mAb or an irrelevant isotype control mAb at the respective optimal antibody concentrations (A: 0.6 µg/ml; B: 2.5 µg/ml). CFUs were determined after incubation for 0, 15, 30, and 60 min. Graphs show the average survival relative to the initial inoculum +/− range from 2 individual experiments.
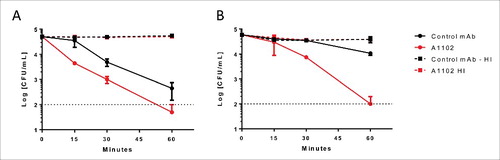
Figure 6. Opsonophagocytotic uptake induced by mAb A1102. RAW264.7 cells were incubated for 30 min at MOI = 1 in the presence of mAb pre-opsonized bacteria and 5% active or heat-inactivated rabbit serum. After the uptake period, gentamicin was added at 100 µg/mL to kill extracellular bacteria. Cells were further incubated for 30 min, and uptake was measured following lysis of cells and plating of surviving intracellular bacteria. Each bar shows the average uptake +/− SEM from 4 individual experiments. Statistical comparison was performed using the Mann-Whitney test.
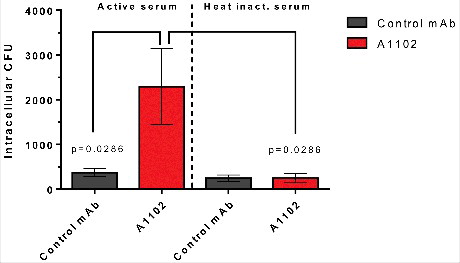
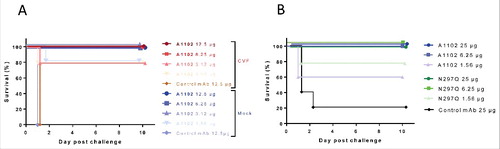
Figure 7. Complement independent protection by A1102. (A) Protective efficacy of A1102 was compared in cobra venom factor-treated (complement depleted) vs mock-treated mice. Groups of 5 mice each received 1 U of CVF (or buffer control) i.p. 30 h before challenge, followed by the indicated doses of A1102 (i.p., 6 h later). Challenge by Kp151 was performed intravenously 24 h after passive immunization with simultaneous GalN sensitization. (B) Efficacy of A1102 compared with its N297Q (aglycosylated) version. Groups of 5 mice were immunized with the indicated amount of antibodies. Challenge by Kp151 was performed intravenously 24 h after passive immunization with mAbs at the time of GalN sensitization. Lethality was monitored daily for 10 d.