Figures & data
Figure 1. Extracellular growth curve of L. pneumophila. Number of viable and culturable L. pneumophila grown in BYE broth was enumerated at every 12 hours using plate count method. BCYE agar plates were used for plate count. Wilcoxon-signed rank test was used to compare the bacterial counts at 12 to 48 hours after inoculation with that at 0 hour. A p-value <0.05 was considered significant.
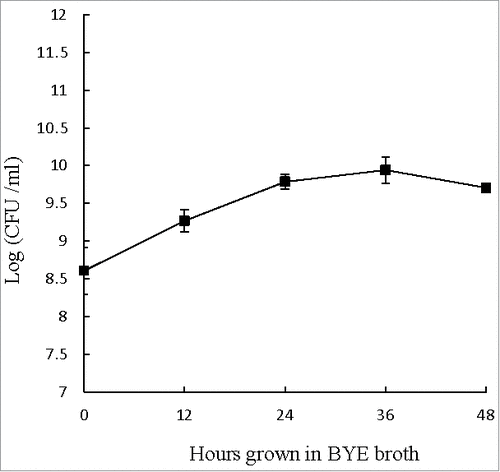
Figure 2. Intracellular growth of L. pneumophila. Viable and culturable L. pneumophila grown in THP-1 and A. castellanii were released by lysis of the host cells at 12-hour intervals up to 48 hours. The released L. pneumophila cells were enumerated using plate count method. BCYE agar plates were used for plate count. Wilcoxon-signed rank test was used to compare the bacterial counts at 12 to 48 hours after inoculation with that at 0 hour. A p-value <0.05 was considered significant.
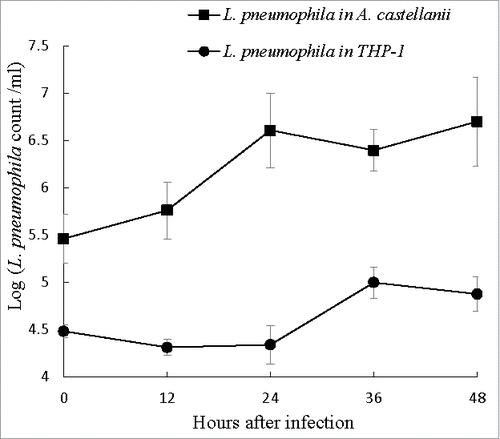
Figure 3. L. pneumophila virulence genes expression during intracellular growth in THP-1 and A. castellanii. L. pneumophila grown in THP-1 (upper panel) and A. castellanii (lower panel) at different time points were collected for RNA isolation and used to run quantitative RT-PCR. All virulence genes had been normalized to housekeeping gene gyrB. Data were expressed as the mean fold change (2−ΔΔCT) compared to T0. Error bars show the SEM (Standard error of the mean). Fold changes at T24 to T48 were statistically compared with that at T12 using Wilcoxon signed rank test, asterisks (#) represent significant differences (p < 0.05).
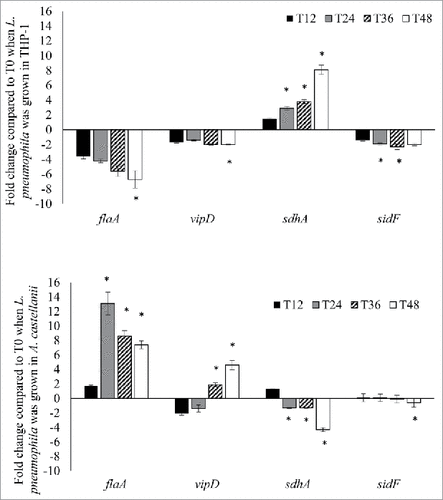
Figure 4. CASP genes expression during L. pneumophila infection in THP-1. Fold changes in the expression of CASP-1 and CASP-3 in THP-1 were detected using quantitative RT-PCR every 12 hours from T0 to T48. Expression levels of the THP-1 genes were normalized to those of its housekeeping gene GAPDH. Data were expressed as the mean fold change. Error bars show the SEM (Standard error of the mean). Fold changes at T24 to T48 were statistically compared with the that at T12 using Wilcoxon signed rank test, asterisks (#) represent significant differences (p < 0.05).
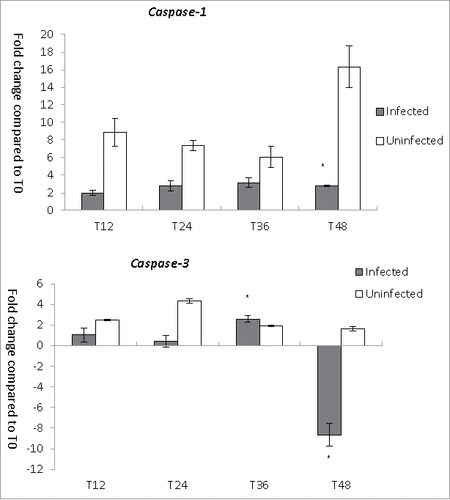
Figure 5. MCASP-1 expression during L. pneumophila grown in A. castellanii that carried change from trophozoites to cysts. Fold changes in the expression of MCASP-1 in A. castellanii were detected using quantitative RT-PCR every 12 hours from T0 to T48 (Left). The expression level of MCASP-1 was normalized to that of A. castellanii 18S rRNA gene. Data were expressed as the mean fold change. Error bars show the SEM (Standard error of the mean). Fold changes at T24 to T48 were statistically compared with the that at T12 using Wilcoxon signed rank test, asterisks (#) represent significant change (p < 0.05). The microscopic images (Right) show the morphologies of the uninfected and infected A. castellanii. Black arrows indicate trophozoites and white arrows indicate cyst forms that have two-layer cell wall (scale bar = 25 μm, magnification 400 ×).
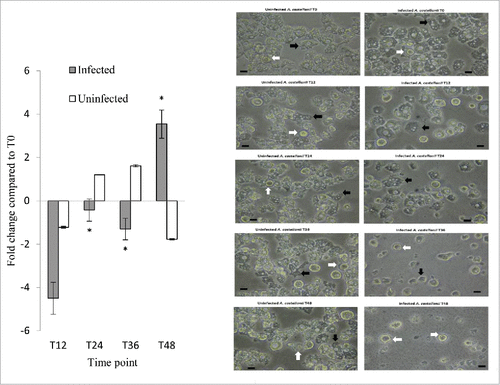
Table 1. Primers and TaqMan probes used in this study. All primers and probes were designed using online Primer-BLAST program (National Centre for Biotechnology Information, US).
Table 2. Flow cytometric analysis of L. pneumophila-infected and uninfected THP-1 cells using annexin-V and PI-staining. THP-1 cells were harvested and stained with annexin-V and PI at 12-hour intervals up to 48 hours. Stained cell samples were analysed on a FACS Aria III flow cytometer and data were analysed using BD FACSDiva software. Paired t-test was used to compared the percentages of cells at various stages between L. pneumophila-infected and uninfected THP-1 cells. Asterisks (#) represent statistical significant differences (p < 0.05).
Table 3. Flow cytometric analysis of L. pneumophila-infected and uninfected A. castellanii cells using annexin-V and PI-staining. A. castellanii cells were harvested and stained with annexin-V and PI at 12-hour intervals up to 48 hours. Stained amoeba samples were analysed on a FACS Aria III flow cytometer and data were analysed using BD FACSDiva software. Paired t-test was used to compared the percentages of cells at various stages between L. pneumophila-infected and uninfected A. castellanii. Asterisks (#) represent statistical significant differences (p < 0.05).
