Figures & data
Figure 1. Binding of H. pylori to the total acid glycosphingolipids of human stomach. Thin-layer chromatogram after detection with anisaldehyde (A), and autoradiograms obtained by binding of H. pylori strain J99 (B), H. pylori strain J99/NAP- (C), and H. pylori strain J99/SabA- (D). The glycosphingolipids were separated on aluminum-backed silica gel plates, using chloroform/methanol/water 60:35:8 (by volume) as solvent system, and the binding assays were performed as described under "Materials and methods”. Autoradiography was for 12 h. The lanes were: Lane 1, total acid glycosphingolipids of human granulocytes, 40 μg; Lane 2, total acid glycosphingolipids of human stomach, 40 μg; Lane 3, total non-acid glycosphingolipids of human stomach from a blood group A individual, 40 μg; Lane 4, reference Leb hexaosylceramide (Fucα2Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer), 4 μg. In lane 2 # denotes the migration of sulfatide, ## the migration of the GM3 ganglioside, ### the migration of the GD3 ganglioside, and #### the migration of the GD1a ganglioside.
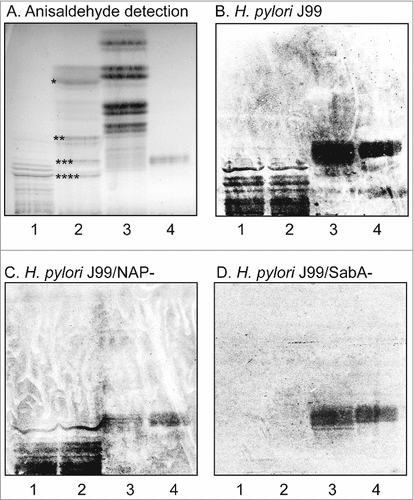
Figure 2. LC-ESI/MS of the total acid glycosphingolipids of human stomach. (A) Base peak chromatogram from LC-ESI/MS of the total acid glycosphingolipids from human stomach. Sulfatide, SO3-3Galβ1Cer; GM3, Neu5Acα3Galβ4Glcβ1Cer; NeuAc-nLc4, Neu5Acα3Galβ4GlcNAcβ3Galβ4Glcβ1Cer; GD3, Neu5Acα8Neu5Acα3Galβ4Glcβ1Cer; GD1a, Neu5Acα3Galβ3GalNAcβ4(Neu5Acα3)Galβ4Glcβ1Cer; GD1b, Galβ3GalNAcβ4(Neu5Acα8Neu5Acα3)Galβ4Glcβ1Cer. (B) MS2 of the ion at m/z 1151 (retention time 17.0 min). (C) MS2 of the ion at m/z 721 (retention time 21.2 min). (D) MS2 of the ion at m/z 758 (retention time 19.4 min). (E) MS2 of the ion at m/z 959 (retention time 22.4 min). (F) MS2 of the ion at m/z 959 (retention time 23.4 min). See Supplemental Figure S1 for interpretation formulas.
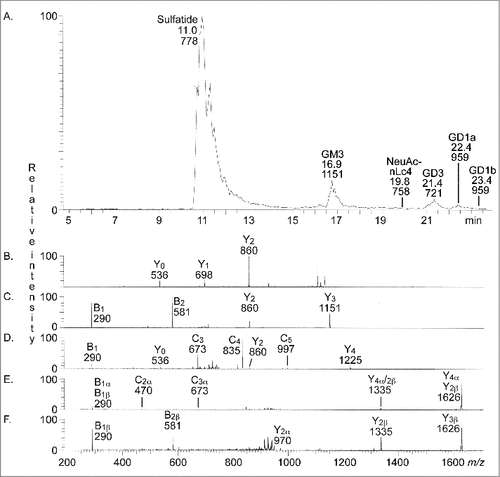
Figure 3. Characterization of the acid glycosphingolipids of human stomach by bacteria and antibody binding. Thin-layer chromatogram after detection with anisaldehyde (A), and autoradiograms obtained by binding of H. pylori strain J99 (B), sulfatide binding CS6 expressing Escherichia coli (C), monoclonal antibodies directed against Neu5Acα3-neolacto (D), the GD1a ganglioside (E), the GD3 ganglioside (F), Neu5Acα3-Lea (G), and Neu5Acα3-Lex (H). The glycosphingolipids were separated on aluminum-backed silica gel plates, using chloroform/methanol/water 60:35:8 (by volume) as solvent system, and the binding assays were performed as described under "Materials and methods”. Autoradiography was for 12 h. The lanes were: Lane 1, total acid glycosphingolipids of human stomach, 40 μg; Lane 2, reference total acid glycosphingolipids of human liver cancer, 40 μg; Lane 3, reference calf brain gangliosides, 40 μg. To allow comparison between the chromatograms in the figures the migration level of NeuAc5α3-neolactohexaosylceramide is indicated by the designation SnLc6.
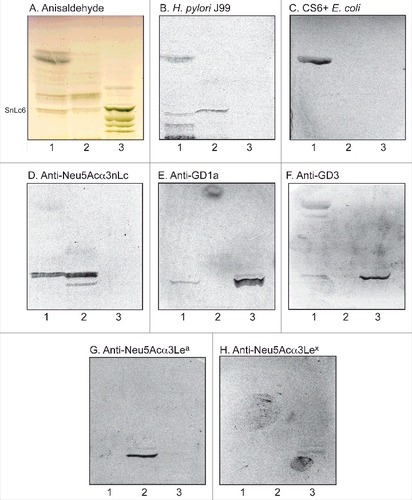
Figure 4. Binding of SabA-expressing H. pylori to acid glycosphingolipid subfractions from human stomach. Thin-layer chromatogram after detection with anisaldehyde (A), and autoradiograms obtained by binding of H. pylori strain J99 (B and C), Solanum tuberosum lectin (D) and monoclonal anti-Neu5Acα3-neolacto antibodies (E). The glycosphingolipids were separated on aluminum-backed silica gel plates, using chloroform/methanol/water 60:35:8 (by volume) as solvent system, and the binding assays were performed as described under "Materials and methods”. Autoradiography was for 12 h. The lanes were on A and B: Lanes 1–6, acid subfractions S1-S6 isolated from human stomach, 4 μg/lane; Lane 7, reference Neu5Acα3-neolactotetraosylceramide (Neu5Acα3Galβ4GlcNAcβ3Galβ4Glcβ1Cer) of human erythrocytes, 4 μg; Lane 8, reference NeuAc-G-10 ganglioside (Neu5Acα3Galβ4GlcNAcβ6 (Neu5Acα3Galβ4GlcNAcβ3)Galβ4GlcNAcβ3Galβ4Glcβ1Cer) of human erythrocytes, 1 μg. The lanes were on C-E: Lane 1, total acid glycosphingolipids of human neutrophils, 40 μg; Lanes 2–4, acid subfractions SI-SIII isolated from human stomach, 1 μg/lane. To allow comparison between the chromatograms in the figures the migration level of NeuAc5α3-neolactohexaosylceramide is indicated by the designation SnLc6.
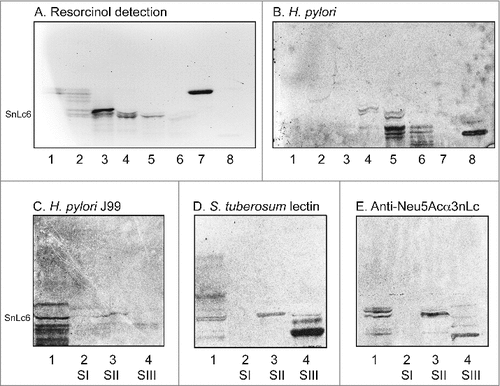
Figure 5. LC-ESI/MS of fraction S-II and fraction S-III of human stomach. (A) MS2 of the ion at m/z 941 (retention time 28.2 min). (B) MS2 of the ion at m/z 1123 (retention time 30.3 min).
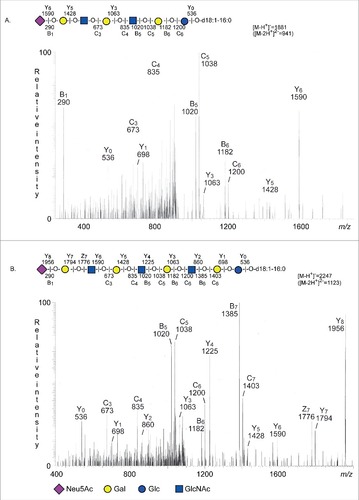
Figure 6 . Binding of SabA-expressing H. pylori to the total acid glycosphingolipids of human gastric mucosa. Thin-layer chromatogram after detection with anisaldehyde (A), and autoradiograms obtained by binding of H. pylori strain J99 (B), and S. tuberosum lectin (C). The glycosphingolipids were separated on aluminum-backed silica gel plates, using chloroform/methanol/water 60:35:8 (by volume) as solvent system, and the binding assays were performed as described under "Materials and methods”. Autoradiography was for 12 h. The lanes were: Lane 1, total acid glycosphingolipids of human neutrophils, 40 μg; Lane 2, total acid glycosphingolipids of mucosal scrapings from human stomach, 40 μg; Lane 3, total acid glycosphingolipids of non-mucosal residue from human stomach, 40 μg; Lane 4, reference NeuAcα3-neolactotetraosylceramide (Neu5Acα3Galβ4GlcNAcβ3Galβ4Glcβ1Cer) of human erythrocytes, 4 μg; Lane 5, reference Neu5Ac-G-10 ganglioside (Neu5Acα3Galβ4GlcNAcβ6(Neu5Acα3Galβ4GlcNAcβ3)Galβ4GlcNAcβ3Galβ4Glcβ1Cer) of human erythrocytes, 4 μg. To allow comparison between the chromatograms in the figures the migration level of NeuAc5α3-neolactohexaosylceramide is indicated by the designation SnLc6.
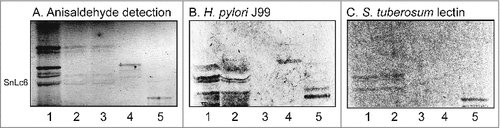
Table 1. Acid glycosphingolipids of human stomach identified by mass spectrometry and binding of carbohydrate recognizing ligands, and results on Helicobacter pylori binding
Table 2. Monoclonal antibodies used in chromatogram binding assays.
