Figures & data
Figure 1. Phylogenetic tree analysis and sequence homology of TFPI-1. (a) From a total of 87 vertebrate species, a phylogenetic tree for the TFPI-1 C-terminal was constructed using Neighbour-Joining tree with 1000 bootstrap replications using the Mega 6 software. (b) Human plasmin and thrombin enzymatic cleavage sites on human TFPI-1 are indicated with arrows. ClustalW multiple sequence alignment of the TFPI-1 C-terminal highlighting amino acids with at least 70% similarity in all species.
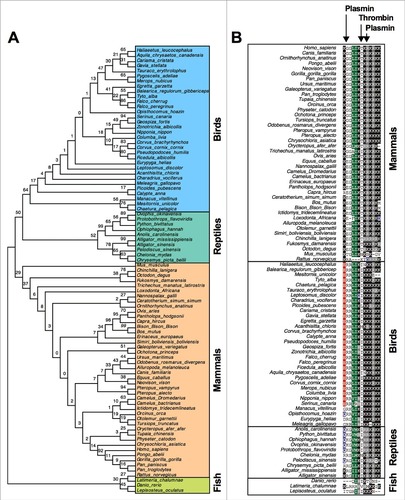
Figure 2. Complement mediated bacterial killing of TFPI-1 C-terminal peptides. (a-b) Bactericidal activity of TFPI-1 peptides was assessed under ex vivo conditions, using viable count analysis. E. coli bacteria were grown to mid-logarithmic phase and incubated with varying concentrations of peptides from mammals, birds, reptiles, and fish species. (a) Peptides were tested either in buffer containing 10 mM Tris, pH 7.4 alone or in presence of 20% citrated plasma from the corresponding host species. (b) Peptides (8 µM) were tested in presence of 20% citrated heat inactivated plasma from the corresponding host species. The bactericidal activity was determined by plating serial dilutions of bacteria on TH agar plates and the number of cfu was counted after overnight incubation. Data are presented as bacterial survival percentage; values are mean ± SD (n = 3) with a non-linear x-axis. (c) TFPI-1 peptides recruit the classical complement pathway.
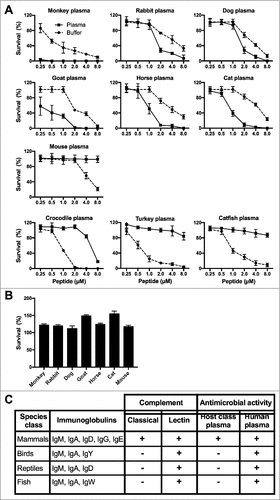
Figure 3. The enhanced bacterial killing by TFPI-1 endogenous C-terminal peptides in human plasma is dependent on immunoglobulins. (a-b, d) 4 µg GGL27 were incubated with 20 µg of human IgM, IgG, and IgG subclasses. In another experiment, 2 µg GGL27 were incubated with 50 µg of human IgG Fab and Fc fragments at indicated time points. GGL27 binding was determined by using rabbit anti-human TFPI-1 C-terminal monoclonal primary antibody and followed by secondary goat anti-rabbit polyclonal antibody (a) binding of GGL27 to IgM and IgG as indicated by arrows (b) binding of GGL27 to different human IgG subclasses (d) binding of GGL27 to Fc fragment at indicated time points. (c) Surface plasmon resonance (SPR) sensorgrams illustrating interactions between GGL27 (analyte) and immobilized IgG (ligand). The curves were obtained after injection of different concentrations of the peptide (125, 25, 500 and 1000 µM respectively) and analysis shows binding incidence with association and dissociation curves between GGL27 and IgG. (e) Biacore graph shows the association and dissociation curve for GGL27 and C1q binding to immobilized IgG. (f-g) ClusPro 2.0 results for the GGL27 peptide (cyan cartoon) docking to the human IgG1. (f) Docking to the homodimeric Fc domain (green and light green cartoon): The top most pose in the figure is at the FcγR site and the middle one in the glycan binding site. The glycan is shown as magenta sticks. The pose on the right hand side is at the hydrophobic patch of the CH2-CH3 domain interface. (g) Docking to the complete IgG1 (lemon green cartoon). The red color regions in the Fc CH2 domain denote the major binding site of C1q globular head. The blue regions denote the amino acids that somewhat restrict the C1q access to the binding site [Citation64]. (h) The importance of GGL27 binding to IgG and mediating complement killing activity was tested via viable count assay. E. coli was incubated for 1 hour with 1 μM GGL27 comparing two different plasma conditions; in normal human citrated plasma or in human citrated plasma pre-treated with IdeS (0.2 µg/µl). Serial dilutions of bacteria were plated on TH agar plates and incubated overnight at 37°C and number of colonies was counted next day. Percentage of E. coli survival when incubating the GGL27 peptide in human citrated plasma alone or in citrated plasma pretreated with IdeS. (i) GGL27 (10 µM) peptide was incubated with fluorescence-labeled E. coli bio-particles for 1 hour in 20% human citrated plasma followed by incubation with murine macrophages (RAW 264.7) for 1 hour at 37°C. Percentage of phagocytosis activity of macrophages was measured by detection of fluorescence using a microtiter plate reader. (j) Plasma samples from three non-pregnant (numbers 1-3) and pregnant (numbers 4-6) women were analyzed by immunoblotting using TFPI-1 C-terminal monoclonal primary antibodies. (k) Endogenously generated TFPI-1 c-terminal peptides inhibit E. coli bacterial growth in pregnant women plasma (left) and this activity was completely abolished when IgG was inactivated with IdeS (right). Plasma from non-pregnant women was used as a control (control n=3, pregnant women n=3).
![Figure 3. The enhanced bacterial killing by TFPI-1 endogenous C-terminal peptides in human plasma is dependent on immunoglobulins. (a-b, d) 4 µg GGL27 were incubated with 20 µg of human IgM, IgG, and IgG subclasses. In another experiment, 2 µg GGL27 were incubated with 50 µg of human IgG Fab and Fc fragments at indicated time points. GGL27 binding was determined by using rabbit anti-human TFPI-1 C-terminal monoclonal primary antibody and followed by secondary goat anti-rabbit polyclonal antibody (a) binding of GGL27 to IgM and IgG as indicated by arrows (b) binding of GGL27 to different human IgG subclasses (d) binding of GGL27 to Fc fragment at indicated time points. (c) Surface plasmon resonance (SPR) sensorgrams illustrating interactions between GGL27 (analyte) and immobilized IgG (ligand). The curves were obtained after injection of different concentrations of the peptide (125, 25, 500 and 1000 µM respectively) and analysis shows binding incidence with association and dissociation curves between GGL27 and IgG. (e) Biacore graph shows the association and dissociation curve for GGL27 and C1q binding to immobilized IgG. (f-g) ClusPro 2.0 results for the GGL27 peptide (cyan cartoon) docking to the human IgG1. (f) Docking to the homodimeric Fc domain (green and light green cartoon): The top most pose in the figure is at the FcγR site and the middle one in the glycan binding site. The glycan is shown as magenta sticks. The pose on the right hand side is at the hydrophobic patch of the CH2-CH3 domain interface. (g) Docking to the complete IgG1 (lemon green cartoon). The red color regions in the Fc CH2 domain denote the major binding site of C1q globular head. The blue regions denote the amino acids that somewhat restrict the C1q access to the binding site [Citation64]. (h) The importance of GGL27 binding to IgG and mediating complement killing activity was tested via viable count assay. E. coli was incubated for 1 hour with 1 μM GGL27 comparing two different plasma conditions; in normal human citrated plasma or in human citrated plasma pre-treated with IdeS (0.2 µg/µl). Serial dilutions of bacteria were plated on TH agar plates and incubated overnight at 37°C and number of colonies was counted next day. Percentage of E. coli survival when incubating the GGL27 peptide in human citrated plasma alone or in citrated plasma pretreated with IdeS. (i) GGL27 (10 µM) peptide was incubated with fluorescence-labeled E. coli bio-particles for 1 hour in 20% human citrated plasma followed by incubation with murine macrophages (RAW 264.7) for 1 hour at 37°C. Percentage of phagocytosis activity of macrophages was measured by detection of fluorescence using a microtiter plate reader. (j) Plasma samples from three non-pregnant (numbers 1-3) and pregnant (numbers 4-6) women were analyzed by immunoblotting using TFPI-1 C-terminal monoclonal primary antibodies. (k) Endogenously generated TFPI-1 c-terminal peptides inhibit E. coli bacterial growth in pregnant women plasma (left) and this activity was completely abolished when IgG was inactivated with IdeS (right). Plasma from non-pregnant women was used as a control (control n=3, pregnant women n=3).](/cms/asset/e690067d-d472-41a0-bc7e-4a4b8f862333/kvir_a_1441589_f0003_oc.jpg)
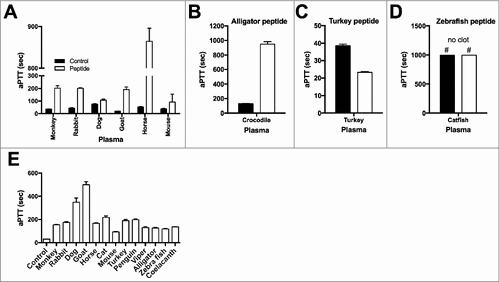
Figure 4. Anticoagulant activities of TFPI-1 C-terminal peptides. Anticoagulant activities of vertebrate TFPI-1 derived peptides (50 µM) in citrated plasma were determined using activated partial thromboplastin time (aPTT). Mammalian peptides were tested in their corresponding host species plasma (a), alligator peptide in crocodile plasma (b), bird peptides in turkey plasma (c) and fish peptides in catfish plasma (d). Note that clotting times > 1000 sec are considered as “no clot”. Data are presented as clotting time in seconds; values are mean ± SD (n = 3). (e) Anticoagulant effects of different vertebrate TFPI-1 peptides were tested in human plasma. Data are presented as clotting time in seconds; values are mean ± SD (n = 3).