Figures & data
Figure 1. Detection of G. intestinalis secreted proteases in the culture supernatant of trophozoites grown alone or in interaction with Caco-2 cell monolayers. Samples of medium were collected after 2 h and 6 h incubation of WB trophozoites at 37°C in DMEM in the absence (A) and presence (B) of Caco-2 monolayer, respectively. The presence of cysteine proteases was detected in concentrated culture supernatants from both axenic and co-cultures by using E64 to inhibit the activities of cysteine protease. For E64 pretreated samples, 100 µM of E64 was used in pre-incubation with ES products at 37°C for 30 min before the electrophoresis. The protein ladders are indicated on the left (kDa).
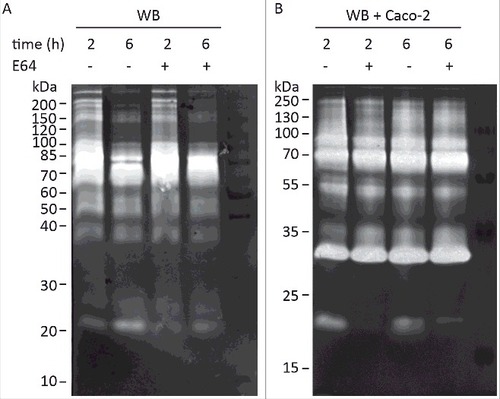
Figure 2. Localization of G. intestinalis cathepsin B-like cysteine proteases. The localization of 3xHA epitope-tagged cathepsin B-like cysteine proteases (CP14109, CP16160 and CP16779) by immunofluorescence microscopy. Transfectants were detected with a mouse anti-HA antibody conjugated to Alexa Fluor 488. Images were taken by a Zeiss Axioplan II Imaging fluorescence microscope and analyzed by ZEN 2.1 software. Bar = 10 µm.
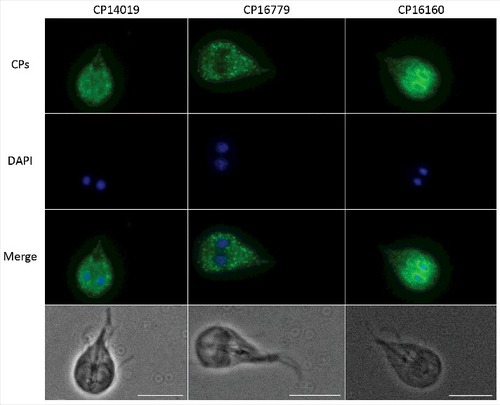
Figure 3. Recombinant G. intestinalis secreted cysteine proteases were expressed, purified and characterized. (A) SDS-page gel showed that both 14019 and 16779 were expressed as the mature form of 28 kDa whereas 16160 was expressed as a combination of a zymogen of 35 kDa and a mature form of 28 kDa. 16160 auto-activated to the mature form after 120 min incubation in sodium acetate buffer (pH 5.5). (B-D) pH profiles of CP14019 (B), CP16779 (C) and CP16160 (D) against the fluorogenic substrates Z-FR-AMC and Z-RR-AMC showed that the pH optimum was 5.5-6.0 for activity against both substrates.
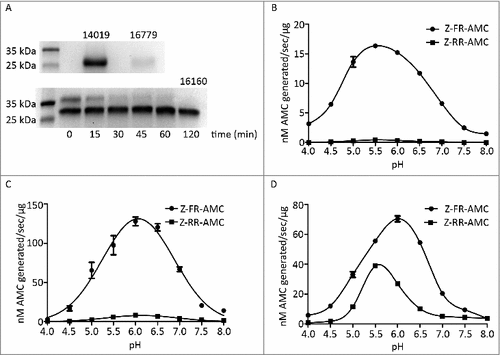
Table 1. Kinetics parameters for secreted cysteine proteases. Recombinant enzymes were incubated with various concentrations of fluorogenic substrates under the optimal pH conditions and fluorescence units were recorded at 37°C over time. Data was calculated using Michaelis-Menten kinetics in the Graphpad Prism 5.0 software.
Figure 4. Effect of ESPs on apical junction complexes. A. Intestinal epithelial cells (Caco-2) were treated with 1 and 5 ug of concentrated ESPs from Giardia WB trophozoites in axenic culture in serum-free medium. The localizations of Zonula Occludens-1 (ZO-1), Occludin and Claudin-1 were studied using specific antibodies. B. Intestinal epithelial cells (Caco-2) treated with non-concentrated ESPs from Giardia trophozoites in axenic cultures in serum-free medium. The localizations of Occludin, Claudin-1, E-cadherin and F-actin were studied using specific antibodies. Images were taken by a Zeiss Axioplan II Imaging fluorescence microscope and analyzed by ZEN 2.1 software.
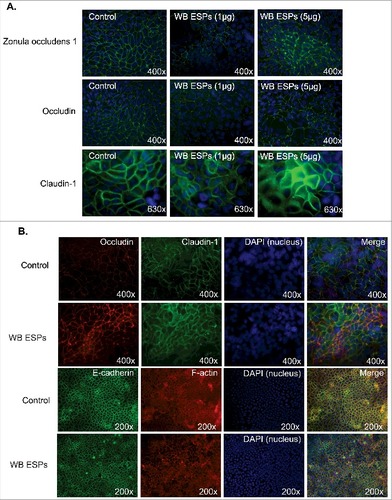
Figure 5. G. intestinalis secreted cysteine proteases have effects on epithelial barrier proteins. A, B, C. Levels of tight junction and adherence junction proteins in Caco-2 whole cell extracts were examined after 24 h of recombinant cysteine proteases treatment (A, 14019; B, 16779; C, 16160), respectively, by Western blot analysis, with GAPDH used as a loading control. Barrier proteins were detected with a mouse or rabbit anti-barrier protein antibody and then incubated with a HR- conjugated mouse or rabbit antibody. D E, F. The localization of occludin in Caco-2 cells visualized by immunofluorescence microscopy. Caco-2 monolayers were incubated with 2.5 µg/ml of 14019 (D), 16779 (E), 16160 (F) in the absence or presence of E64 (10 µM) for 24 h, followed by fixation of PFA. Occludin was detected with a mouse anti-occludin antibody and then incubated with an Alexa Fluor 488-conjugated mouse antibody. Images were taken by a Zeiss Axioplan II Imaging fluorescence microscope and analyzed by ZEN 2.1 software. Bar = 50 µm.
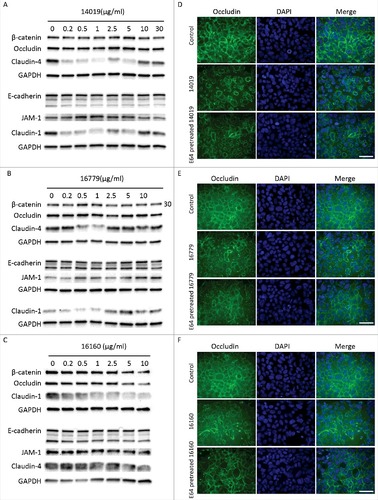
Figure 6. G. intestinalis ESPs and cysteine proteases have a proteolytic effect on chemokines. A. Digestion of chemokines with ESPs collected from axenic Giardia WB cultures. B-D. Cleavage of chemokines by recombinant cysteine proteases was performed as described in Methods. 100 µM of E-64 was routinely added to the proteases for 30 min at 37°C. Samples were analyzed by 16.5% Tris-Tricine gel under reducing condition. ##represents “clear degradation of chemokines”, #represents “uncertain cleavage of chemokines”.
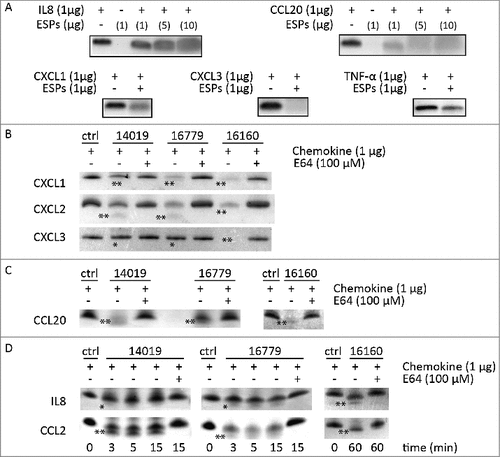
Figure 7. Model of cathepsin B cysteine proteases function during Giardia-host cells interactions. Giardia cathepsin B cysteine proteases released during host-parasite interaction have proteolytic activity and are capable of destroying the junctional complexes (TJs and AJs). The CPs can pass the epithelial barrier and degrade chemokines that are induced by intestinal epithelial cells and released on the basolateral side in response to Giardia infection.