Figures & data
Table 1. Serogroup A Neisseria meningitidis capsule locus mutants (n = 13) and reference isolates (n = 3) and properties of their capsular polysaccharide.
Figure 1. Flow cytometric analysis of the binding of the anti-A capsule specific mAb 95/674 (A) and of mouse anti-ACWY capsule antiserum to meningococcal cells (B). The fluorescence intensity of fixed bacterial cells was determined (counts). Isolate 2602: reference isolate; isolate 1976: no capsule expression in the mutant carrying a non-synonymous SNP in the ctrE gene responsible for translocation of the polysaccharide; isolate 2008: increased capsule expression in the mutant carrying a non-synonymous SNP in the csaB gene that codes for a capsule polymerase; isolate 1666: lack of binding of antibodies to the mutant carrying a stop mutation in the csaC gene that encodes an O-acetyltransferase.
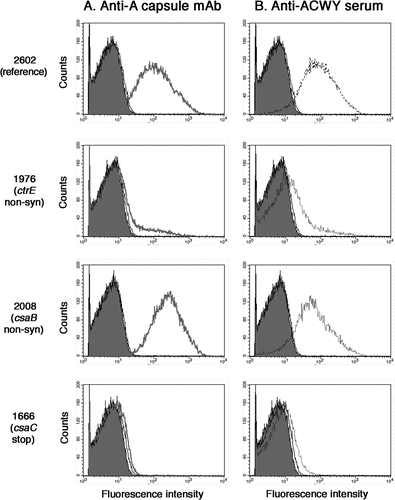
Figure 2. HR MAS-NMR spectra (from 5.2 to 5.8 ppm characteristic for H1 signals of O-acetylated (OAc) and not O-acetylated (deOAC) sugar units indicated in the structure of the serogroup A polysaccharide repeating unit of three selected isolates compared to the reference isolate 2602. No capsule expression in isolate 1976 carrying a non-synonymous SNP in the ctrE gene responsible for translocation of the polysaccharide; similar O-acetylation level with respect to the reference in isolate 2008 carrying a non-synonymous SNP in the csaB gene that codes for a capsule polymerase; no O-acetylation in isolate 1666 carrying a stop mutation in the csaC gene that encodes an O-acetyltransferase.
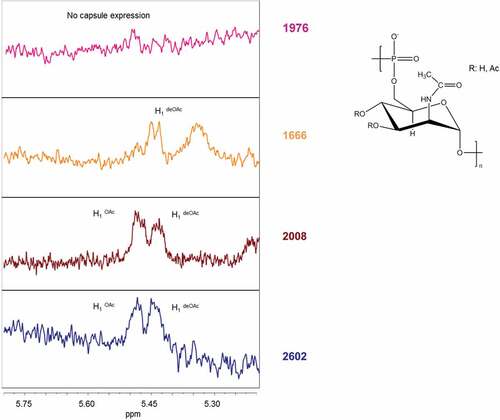
Figure 3. Effect of point mutations in the capsule synthesis locus on bactericidal activity mediated by anti-ACWY capsule antiserum and anti-LPS mAb. Typical results are shown for selected reference and mutant isolates. Reference isolate 2602: sensitive against both anti-ACWY capsule antiserum and anti-LPS mAb; isolate 1976 lacking capsule due to a non-synonymous SNP in the ctrE gene: lack of activity of anti-ACWY capsule antiserum; isolate 2008 expressing more polysaccharide than the reference isolate as a result of a non-synonymous mutation in the csaB gene: generally reduced bactericidal effect irrespective of the specificity of the antibodies; isolate 1666 expressing non-O-acetylated capsular polysaccharide: lack of activity of anti-ACWY capsule antiserum. Error bars represent standard error of the mean titer of triplicate measurements in three independent experiments. The bottom dotted line indicates the lowest serum dilution (1:10) and highest mAb concentration (100 µg/mL) tested.
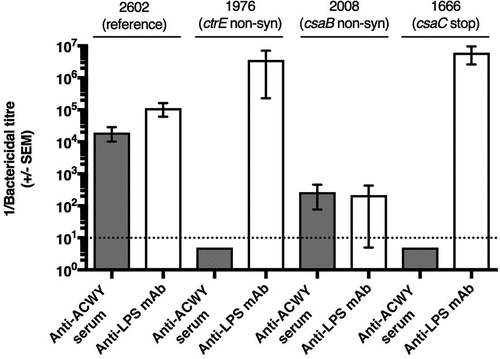
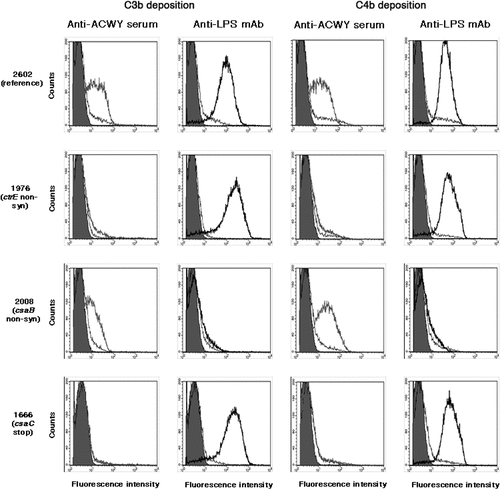
Figure 4. Flow cytometric analysis of the deposition of human C3b and C4b on fixed bacterial cells. Anti-ACWY capsule antiserum (diluted 1:50) or anti-LPS mAb EI9.2 (4 µg/mL) was used together with 5% IgG depleted human serum to investigate the effect of the mutations on antibody mediated C3b and C4b deposition. Typical results are shown for one of the reference isolates and for selected mutant isolates. The fluorescence intensity of fixed bacterial cells was determined (counts). Grey filled area: cells only incubated with secondary antibody; black thin line: negative control with 4 µg/mL of an unrelated IgG1 mAb; grey thick line: anti-ACWY capsule antiserum; black thick line: anti-LPS mAb. Reference isolate 2602: C3 and C4 deposition is caused both by anti-capsule and anti-LPS antibodies; isolate 1976 lacking capsule due to a non-synonymous SNP in the ctrE gene: no C3 and C4 deposition with anti-capsule antibodies; isolate 2008 expressing more polysaccharide than the reference isolates as a result of a non-synonymous mutation in the csaB gene: C3 and C4 deposition by anti-LPS mAb is strongly reduced; isolate 1666 expressing non-O-acetylated capsular polysaccharide: no C3 and C4 deposition caused by polyclonal anti-ACWY capsule antiserum.