Figures & data
Figure 1. Schematic view of immune responses to T. gondii upon initial infection. Cells involved in the innate and adaptive immune response to T. gondii infection are shown. Initial host control of parasite infection induces the production of the pro-inflammatory cytokine interleukin 12 (IL-12) by macrophages and dendritic cells. IL-12 will in turn activate natural killer (NK) and T cells to secrete interferon γ (IFN-γ). Neutrophils and T cells also produce IFN-γ in response to infection. IFN-γ then activates several host defense mechanisms for intracellular elimination of T. gondii, including the activation of interferon-induced GTPases, and the induction of nutrient and oxidative stresses. Activated B cells can also help limiting the spread of the parasites to some extent.
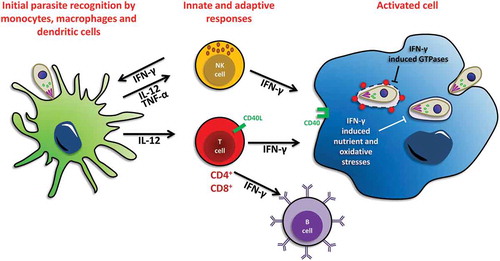
Figure 2. Autophagy-related protein LC3 associates with different types of membranes. (a) Schematic representation of the mammalian autophagic process and its regulatory machinery. The process is initiated by the formation of a structure called the phagophore, which engulfs cytoplasmic cargo and, once complete, will form a double membrane compartment called the autophagosome. The autophagosome will then fuse with lysosomes to form an autolysosome where the autophagic cargo will be degraded for subsequent recycling. The whole process is regulated by upstream kinases such as Target of Rapamycin (TOR) and class III phosphatidylinositol 3-kinase (PtdIns3K). Microtubule-associated protein 1 Light Chain 3 (LC3), an important player for autophagosome formation, is conjugated to phosphatidyl ethanolamine (PE) on the membrane of elongating phagophores thanks to ubiquitin-like conjugation systems. (b) The process of LC3-associated phagocytosis (LAP) is involved in the degradation of extracellular pathogens. LC3 associates with phagosomal membranes thanks to the same ubiquitin-like conjugation machinery required for LC3 membrane conjugation to the phagophore during canonical autophagy. The emerging phagosome, now referred to as LAPosome, will mature by fusion with lysosomes and the cargo will be degraded.
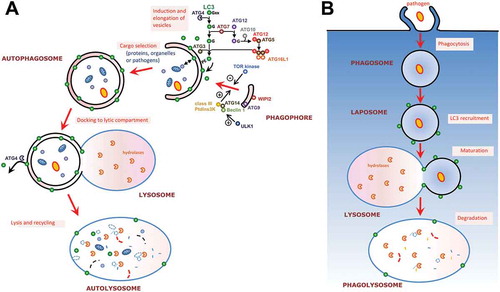
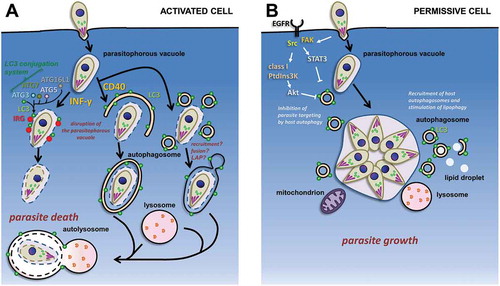
Figure 3. Schematic representation of the interplay between host autophagy-related machinery and intracellular tachyzoites. (a) Possible autophagy proteins-dependent pathways for the degradation of tachyzoites in immunologically-activated mouse host cells. The LC3-centered autophagy machinery has an IFN-γ-stimulated non-canonical role in the recruitment of immunity-related GTPases (IRGs) to the parasitophorous vacuole; on the other hand, CD40 stimulation leads to parasite elimination by canonical autophagy, LAP, or a related lysosome-dependent mechanism. (b) In permissive host cells, invading tachyzoites can potentially block the autophagy machinery of the host by interfering with epidermal growth factor receptor (EGFR)-dependent signaling pathways. Developing parasites can also recruit host organelles, including autophagic vesicles, to access nutrients sources.