Figures & data
Figure 1. S. suis NT is able to convert dAMP to dAdo. (a), The concentration of inorganic phosphate (IP) released from dAMP in the tNT group (96.09 μM) was significantly higher than that of IP in the GroEL group (41.61 μM) or working buffer group (23.69 μM). Statistical significance was examined by one-way ANOVA and Dunnett’s multiple comparisons test. ** P < 0.01; n = 3. The generation of dAdo by NT was detected by RP-HPLC analysis. The arrow indicates the dAdo produced. Samples are from tNT with dAMP group (b), the working buffer group (c), and the standard sample of dAdo (d).
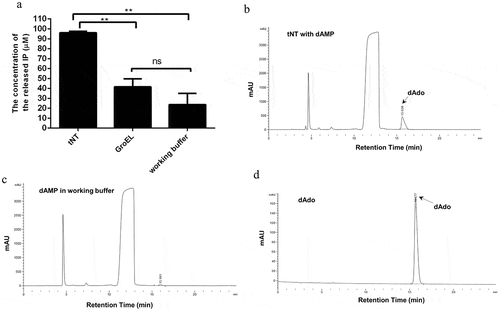
Figure 2. dAdo generated by tNT triggers caspase-3-dependent death of mouse macrophage RAW 264.7 cells. (a), In all the groups in the presence of 100 μM dCF (orange columns), the viability of RAW 264.7 cells in the tNT+ dAMP group (65.66%, 63 mM dAMP pretreated with 6 μM tNT at 37°C for 2 h) was significantly lower than that of cells in the H2O (95.33%), 6 μM tNT (86.00%), 63 mM dAMP (76.33%), or working buffer (81.33%) groups. There was no significant difference of cell viability between the tNT+ dAMP group and the 55 μM dAdo group (66.66%, as a positive control) in the presence of dCF. A significant difference of cell viability was observed in tNT+ dAMP groups (with or without dCF) and dAdo groups (with or without dCF). The viability of RAW 264.7 cells was measured by the trypan blue staining. Statistical significance was examined by one-way ANOVA and Dunnett’s multiple comparisons test. ns, not significant; *** P < 0.001; n = 3. (b), Western blot analysis showed that the band of active caspase-3 was detected in RAW 264.7 cells treated with 55 μM dAdo and 100 μM dCF for 12 h, 16 h, or 24 h. Jurkat cell extract treated with cytochrome c in vitro, obtained from Cell Signaling Technology (#9663), was used as a positive control. (c), The band of active caspase-3 was also detected in RAW 264.7 cells treated with dAMP that had been incubated with tNT in the presence of dCF for 12 h. β-actin was used as a loading control. Experiments were done in triplicate.
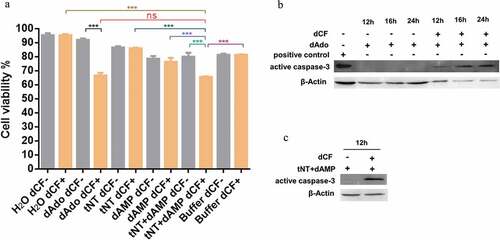
Figure 3. The detection of Ado and dAdo in vivo. The abundance of Ado and dAdo in plasma was determined by RP-HPLC analysis. (a), The concentration of plasma dAdo from mice infected with the WT strain was 0.761 μg/mL, while the concentration of plasma dAdo from mice inoculated with Δnt or PBS was below the method detection limit (0.019 μg/mL). (b), The amount of plasma Ado from mice infected with the WT strain (0.5194 μg/mL) was significantly higher than that of plasma Ado from mice injected with Δnt (0.3584 μg/mL) or PBS (0.2321 μg/mL). The RP-HPLC chromatograms of above samples are shown in Supplementary Figure S3. Statistical significance was examined by one-way ANOVA and Dunnett’s multiple comparisons test. ns, not significant; *P < 0.05; n = 4.
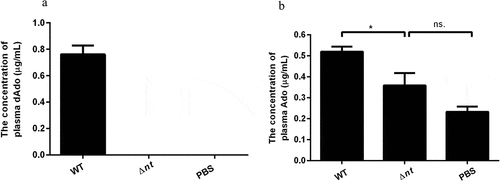
Figure 4. S. suis NT contributes to dampening immune responses. (a), NT causes monocytopenia in mouse blood in vivo. The number of monocytes in blood from mice infected WT strain (3.2×107/L) was significantly less than that of monocytes in blood from mice infected with Δnt (5.2×107/L). Statistical significance was examined by one-way ANOVA and Dunnett’s multiple comparisons test. *P < 0.05; n = 5. (b), In vivo transcriptome analysis in mouse blood. Compared with blood from mice infected with the WT strain GZ0565, 320 genes were downregulated and 88 genes were upregulated in blood from mice infected with Δnt. The top five enrichment GO categories based on biological process for the total 408 differentially expressed genes belonged to immune response, chemotaxis, inflammatory response, innate immune response, and neutrophil chemotaxis.
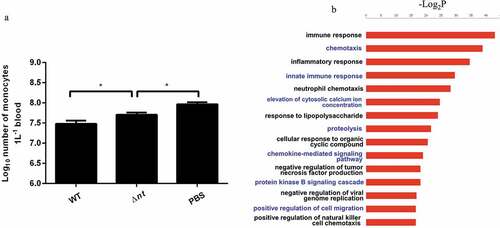
Table 1. The key DEGs in blood from mice between WT infection group and Δnt infection group.
Figure 5. S. suis synthesizes dAdo and Ado by NT to dampen host immune responses. S. suis NT increases the concentration of dAdo and Ado in mouse blood in vivo. dAdo causes monocytopenia in mouse blood in vivo. In addition, the inhibitory effect of NT on immune responses and neutrophil functions in vivo may be mediated through the generation of Ado; the inhibitory effect of Ado on neutrophil functions through occupancy of A2a receptor was demonstrated by Liu et al. in vitro [Citation15]. The dot line means the directly inhibitory effect of Ado on immune responses and neutrophil functions in vivo needs to be further validated.
![Figure 5. S. suis synthesizes dAdo and Ado by NT to dampen host immune responses. S. suis NT increases the concentration of dAdo and Ado in mouse blood in vivo. dAdo causes monocytopenia in mouse blood in vivo. In addition, the inhibitory effect of NT on immune responses and neutrophil functions in vivo may be mediated through the generation of Ado; the inhibitory effect of Ado on neutrophil functions through occupancy of A2a receptor was demonstrated by Liu et al. in vitro [Citation15]. The dot line means the directly inhibitory effect of Ado on immune responses and neutrophil functions in vivo needs to be further validated.](/cms/asset/ed59d762-8e8a-4921-8bd8-0429a4457cd9/kvir_a_1520544_f0005_c.jpg)
