Figures & data
Figure 1. The necessity of Pep27 for virulence. (a) The target gene(s) were deleted and replaced by the erythromycin resistance cassette ermB in an orientation opposite to the direction of transcription by homologous recombination to prevent a polar effect. (b) A549 cells were infected with the mutants, and cytotoxicity was determined using the lactate dehydrogenase (LDH) assay. (c). Mice (CD1, n = 10 per group) were infected by the intranasal (i.n.) administration of 1–2 × 107 CFU of the WT D39 or its isogenic mutants, and the survival rates were determined. (d) The WT D39 and various isogenic mutants were infected by i.n. administration, and the number of viable cells was determined 24 or 48 h post-infection. Except (a), all the data are expressed as the mean ± standard error of mean (SEM) of 3 experiments performed in duplicate. *P < 0.05 (one-way ANOVA).
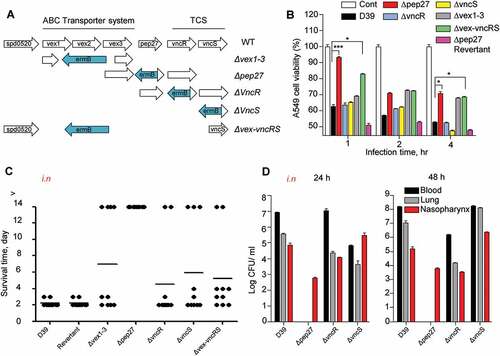
Figure 2. Serum-dependent inductionof vncR/S and autophosphorylation of VncS. (a–d) Wild type (WT) D39 cells were cultured in THY broth up to the mid-log phase (OD550 = 0.3), and then incubated with human serum for 5 min (a, c) or with 10% human serum for the durations specified (b, d). The mRNA levels were analyzed by qRT-PCR (a, d), and the Pep27 levels were determined by analyses with western blotting (c, d). (e) WT D39 cells at the mid-log phase were exposed to 10% human serum for the specified durations, and the protein levels were determined by analysis with western blotting. (f) The VncS expressed in E. coli (pHis-VncS) cells or the empty plasmid vector without any insert (Vec, control) was used for the auto-phosphorylation assays in the presence of [γ-32p] ATP. The loading dose of the VncS protein was measured by immunoblotting assays using anti-His6 antibody. (c, d, e, f) Representative data of at least 3 independent experiments. (a, b) The data are expressed as the mean ± standard error of the mean (SEM) of 3 experiments performed in quadruplicate. *P < 0.05 (one-way ANOVA).
![Figure 2. Serum-dependent inductionof vncR/S and autophosphorylation of VncS. (a–d) Wild type (WT) D39 cells were cultured in THY broth up to the mid-log phase (OD550 = 0.3), and then incubated with human serum for 5 min (a, c) or with 10% human serum for the durations specified (b, d). The mRNA levels were analyzed by qRT-PCR (a, d), and the Pep27 levels were determined by analyses with western blotting (c, d). (e) WT D39 cells at the mid-log phase were exposed to 10% human serum for the specified durations, and the protein levels were determined by analysis with western blotting. (f) The VncS expressed in E. coli (pHis-VncS) cells or the empty plasmid vector without any insert (Vec, control) was used for the auto-phosphorylation assays in the presence of [γ-32p] ATP. The loading dose of the VncS protein was measured by immunoblotting assays using anti-His6 antibody. (c, d, e, f) Representative data of at least 3 independent experiments. (a, b) The data are expressed as the mean ± standard error of the mean (SEM) of 3 experiments performed in quadruplicate. *P < 0.05 (one-way ANOVA).](/cms/asset/82811273-a7ea-4a08-843a-860132ec9a01/kvir_a_1526529_f0002_oc.jpg)
Figure 3. Pep27 is essential for lysis and secretion of proinflammatory cytokines. (a–d) Serum and/or lysis inducers were added to the cultures of WT D39 (a, b) or Δpep27 (c, d) grown in THY broth in the early exponential phase, and the OD was monitored. When required, the lysis inducers, deoxycholate (DOC, 100 µg ml−1) and vancomycin (0.4 µg ml−1), were added. (e) The WT D39 cells in the mid-log phase were exposed to 10% human serum for the specified durations, and the cell culture supernatant containing the released chromosomal DNA was detected by PCR using 16S rRNA primers. (f, g) CD1 mice (n = 3 per group) were infected by intravenous (i.v.) administration of either the WT D39 cells (7 × 107 CFU) or mutant D39 Δpep27 cells (1 × 108 CFU). The blood samples were differentially centrifuged to separate the bacteria bound to the host cells (pellet) from the unbound bacteria collected in the supernatant, and the number of bacteria were enumerated by plating on blood agar (F). The chromosomal DNA released into the supernatant was detected by PCR using 16s rRNA primers (g). (h–j) Balb/c mice (n = 7 per group) were infected with 2 × 108 CFU of the WT pneumococcal strain and the lung homogenates were used for determining the cytokine levels by ELISA. (k) CD1 mice were intratracheally infected with the WT D39 strain (1 × 107 CFU) or the D39 mutant Δpep27 (2 × 108 CFU). Lung sections at 1 or 2 days post-infection were stained with hematoxylin and eosin. All the data are expressed as the mean ± standard error of the mean (SEM) of a minimum 3 of independent experiments except (g) (a–d, error bars are smaller than the symbols used here). *P < 0.05 (a–d, f: one-way ANOVA, h–j: two-way ANOVA). (k) Representative data of at least 3 independent experiments.
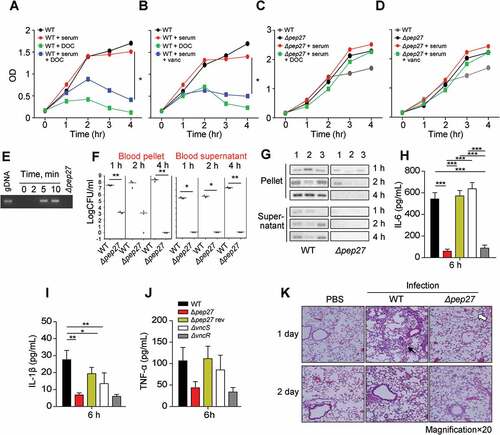
Figure 4. Infection with Δpep27 abolishes virulence as well as invasion into immunocompromised mice. (a) The attenuated virulence of Δpep27 in the brain. CD1 mice (n = 5) were infected with the WT D39 (2 × 104), mutant Δpep27 (pep27 1 × 106), or R6 (2 × 106) strains via intracranial (i.c.v) administration, and the survival times were determined. (b) The rapid clearance of Δpep27 observed in the brain. CD1 mice (n = 3) were infected with the WT D39 (5 × 103), mutant Δpep27 (1 × 104), or R6 (7 × 103) strains via i.c.v. administration, and the number of viable cells was determined. (c, d) Nude (T-cells deficient) or SCID (both B- and T-cell deficient) mice (n = 5) were infected by intraperitoneal (i.p.) administration of 1 × 104 CFU (c), or intranasal (i.n.) administration of 1 × 107 CFU of Δpep27 (d), and survival times were determined. Each data point represents one mouse. The data are representative of 3 (a, b) or 2 (c, d) independent experiments. *P < 0.05 (one-way ANOVA) as compared between groups.
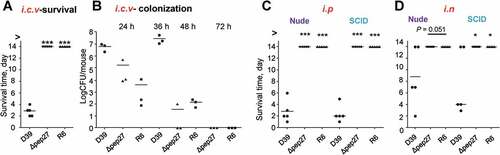
Figure 5. The VncS ligand, lactoferrin (LF), induces vncR/S, Pep27, and lysis. (a) Purified His6-VncS protein bound to a 3-dimensional Sol-gel® bed was incubated with human serum for screening VncS ligands. The bound ligand was subsequently subjected to SDS-PAGE and mass analyses. (b–d) The WT D39 strain was incubated at the mid-log phase with various concentrations of LF for 5 min (b, c) or with 30 mM LF for the specified duration (d). Western blotting (b) and qRT-PCR (c) were performed. Quantification of the released chromosomal DNA using the genomic DNA (gDNA) as a control (d). (e) The WT D39 strain was incubated for 5 min with LF in the LF-depleted human sera, which was prepared by adding either 1 or 5 μg of LF antibody to human serum. The mRNA levels were determined by qRT-PCR. The data in (a), (b), and (d) are representative of at least 3 independent experiments. The data in (C) and (e) are expressed as the mean ± standard error of the mean (SEM) of 3 experiments performed in quadruplicate. *P < 0.05 (one-way ANOVA).
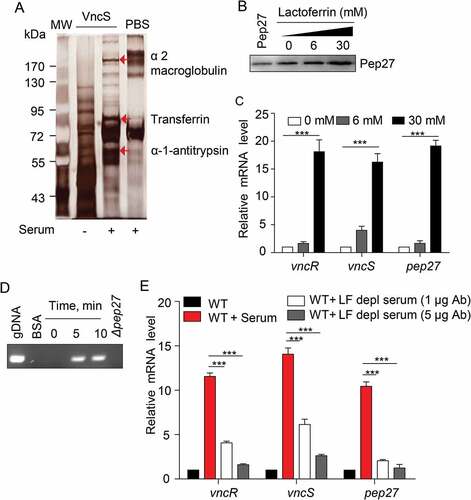
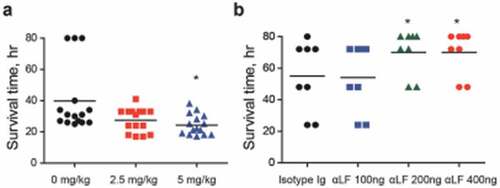
Figure 6. Lactoferrin (LF)-dependent sepsis. (a) LF was orally administered to mice (n = 15 per group), 6 h prior to lethal intraperitoneal (i.p.) infection with the WT D39 strain (2 × 104 CFU), and survival rates were recorded every hour. (b) LF antibody was administered to mice (n = 8 per group) by i.p. administration at doses of 100, 200, and 400 ng/mouse and by i.p. route usingone-fourth of the dose used during intranasal (i.n.) administration at 25, 50, and 100 ng/mouse 24 h prior to the i.n. administration of the WT D39 strain (2 × 108 CFU), and the survival rates were monitored. The data are expressed as the mean ± standard error of the mean (SEM) of 3 experiments performed in duplicate. *P < 0.05 (Log-rank test).