Figures & data
Figure 1. Diagramatic representation of the similarities between invertebrate Toll signalling and vertebrate toll-like signalling.
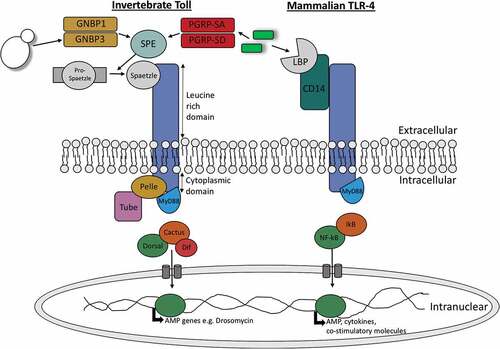
Table 1. A comparison of the antimicrobial peptides present in insects and humans.
Table 2. A comparison of humoral receptors, anti-microbial peptides, cascades and enzymes in mammalian and insect humoral immune responses.
Figure 2. Comparision of insect IMD pathway and mammalian TNF-α pathway.
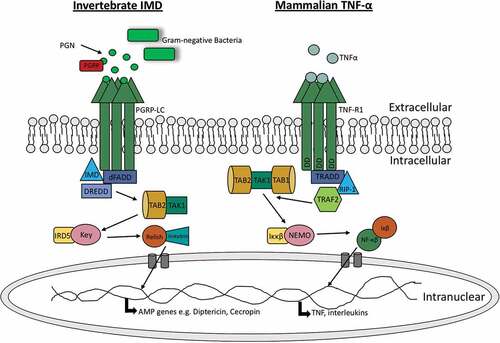
Figure 3. Sequence comparison between human (h) von Willebrand factor and Drosophila (d) Hemolectin protein sequences using emboss needle pairwise sequence alignment. Highlighted in yellow are conserved cysteine residuals. hVWF and dHemolectin are up to 30.9% similar protein sequences. * (asterics); indicates a single, fully conserved residue,: (colon); indicates conservation between groups of strongly similar properties, . (full stop); denotes conservation between groups of weak similar properties).
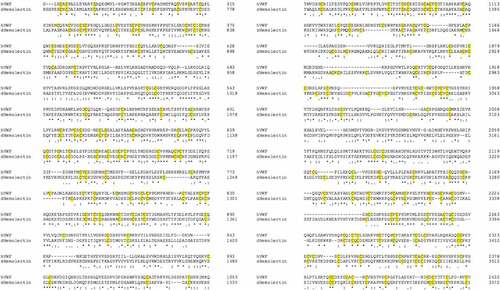
Figure 4. Schematic comparison of the hemolymph/blood clotting system in insects versus mammals.
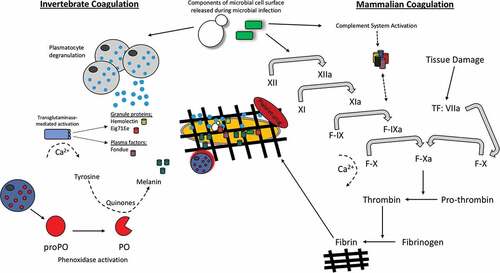
Figure 5. Schematic diagram of the proPO-system for melanin production in insects.
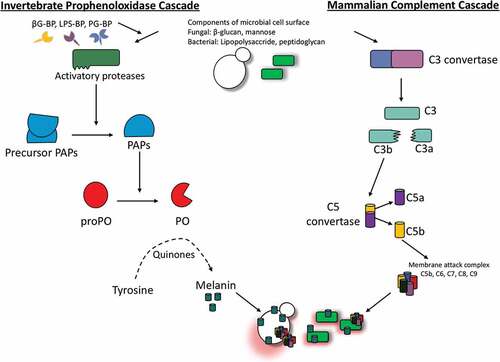
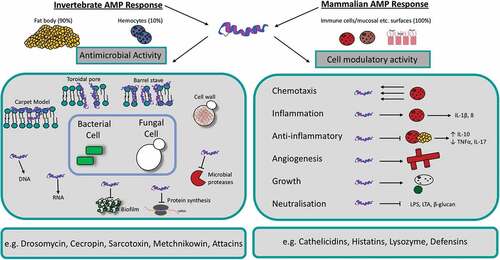
Figure 6. Schematic diagram of the mechanism of action and function of antimicrobial peptides in insects and mammals.