Figures & data
Table 1. Summary of arbovirus and autophagy pathway.
Table 2. Summary of arbovirus and apoptosis pathway.
Table 3. Summary of arbovirus and UPR pathway.
Table 4. Summary of influenza virus infection and autophagy pathway.
Table 5. Summary of influenza virus infection and apoptosis pathway.
Table 6. Summary of influenza virus infection and UPR pathway.
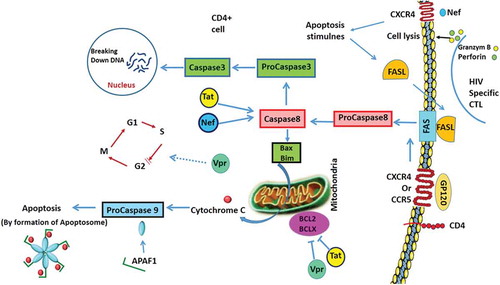
Table 7. Summary of HIV infection and autophagy, apoptosis and UPR pathways.
![Figure 1. Autophagy Signaling During Arbovirus Infection.There are five possible mechanisms for modulating viral replication which include: a) some arboviruses such as DENV and JEV can use amphisome formation for their entry and replication; b) several arboviruses such as DENV, ZIKV, JEV, CHIKV and TBEV exert diverse mechanisms to induce autophagosome formation to enhance viral replication/translation complexes. DENV is associated with NS4A in up-regulating PI3K-dependent autophagy. CHIKV induces the IRE1α–XBP-1 pathway in conjunction with ROS-mediated mTOR inhibition; c) DENV benefits from autophagy activation by using lipid droplets as an energy source for replication; d) Viruses such as DENV-2 and CHIKV can increase their replication by prolonging cell survival and preventing cell death; and d) VSV appears to suppress IFN signaling by conjugated Atg5-Atg12, leading to an effective virus-suppressing immune response [modified from [Citation131]] . DENV: Dengue virus; ZIKV: Zika virus; JEV: Japanese encephalitis virus; CHIKV: Chikungunya virus; TBEV: tick-borne encephalitis virus; VSV: vesicular stomatitis virus.](/cms/asset/7e09a485-2a61-4926-9b0d-62fb5b5a6fc5/kvir_a_1605803_f0001_oc.jpg)
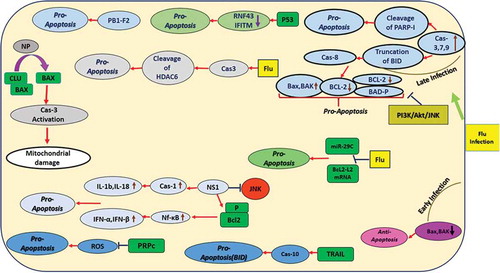
![Figure 2. Apoptosis Signaling During Arbovirus Infection.Arboviruses exert their effect on apoptosis through different signaling routes. A mechanism for anti-apoptotic activity by these viruses is up-regulation of the PI3K signaling pathway. Another mechanism that viruses can regulate is the initiation of protein 14–3-3 through activation of JNK followed by induction of PKR. CCHFV replication is associated with upregulation of Bax, HRK, PUMA, and Noxa. WNV, JEV and DENV block or delay apoptosis via activating PI3K/Akt signaling. WNV can trigger apoptosis after several rounds of replication through caspases-3 and −12 and p53. JEV triggers ROS-mediated ASK1-ERK/p38 MAPK activation which leads to initiation of apoptosis. JEV may affect Bcl-2 expression to increase anti-apoptotic response. DENV may subvert apoptosis by inhibiting NF-kB. DENV reduces immune responses by activation of p53-dependent apoptosis. RVFV inhibits caspase-8 to regulate pro-apoptotic p53 signaling. The BTV-induced apoptosis involves NF-kB [modified from [Citation131]]. DENV: Dengue virus; ZIKV: Zika virus; WNV: West Nile virus; JEV: Japanese encephalitis virus; CHIKV: Chikungunya virus; CCHFV: Crimean Congo hemorrhagic fever virus; RVFV: Rift Valley fever virus; BTV: bluetongue virus.](/cms/asset/0e6e7659-c0c6-46f2-849f-3eea58424224/kvir_a_1605803_f0002_oc.jpg)
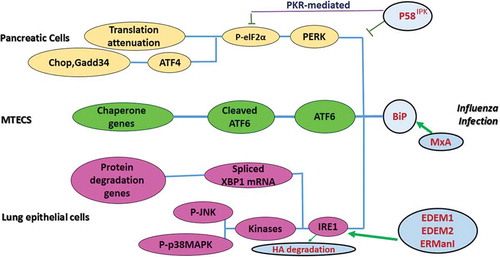
![Figure 3. UPR Signaling During Arbovirus Infection.ER stress is enhanced in viral infected cells and activates UPR proteins (e.g. PERK, ATF6, and IRE1). Activated PERK induces ATF4 via phosphorylation of eIF2α, causing attenuation of translation and genes encoding CHOP. Upon IRE1 activation, TRAF2 and XBP mRNA1 splicing are initiated in the cytoplasm, which subsequently leads to regulation of UPR target genes. The degradation of ATF6 is increased through recruitment of ATF6, a UPR sensor, which results in the regulation of protein folding. The consequences of UPR activation are necessary for viral replication and pathogenesis [modified from [Citation131]].](/cms/asset/b3d27685-0d52-4014-ba42-7dfc74dfe501/kvir_a_1605803_f0003_oc.jpg)
![Figure 4. Autophagy Signaling During Influenza A Virus Infection.Influenza A virus (IAV) induces the NLRP3 inflammasome, which causes mitochondrial damage and release of ROS, which prevents the conversion of LC3-II to LC3-I by degrading Atg4 and leads to increased levels of LC3-II. NLRP3 forms an inflammasome complex with ASC and induces the production of inflammatory cytokines. IAV also binds to Beclin1 by the viral M2 protein. It up-regulates the expression of several autophagy-related genes, which can increase autophagic flux. M2 also contains an LC3-interacting region (LIR) which is required for influenza virus subversion of autophagy; this leads to LC3 redistribution to the plasma membrane in infected cells. The complex P-mTORC2/p70S6K blocks lethal autophagy. Autophagosome formation blocks IFN-β and reduces ISG expression [modified from [Citation337]].](/cms/asset/234dc535-6d3c-4abc-bbd1-8c2e9e1649a2/kvir_a_1605803_f0004_oc.jpg)