Figures & data
Table 1. A. fumigatus strains utilized in this study.
Table 2. Oligonucleotides used for genetic manipulation of A.fumigatus.
Figure 1. Protein geranylgeranylation promotes A. fumigatus thermotolerant growth.
Conidia (5x103) obtained from control (Ctrl), Δcdc43 or complemented (Δcdc43:cdc43) strains were spot inoculated (5 µl) onto GMM (A), YPD (C), or PDA (D) agar. Growth and development of colony morphology at 27ºC, 37ºC, and 45ºC were monitored and images represent growth and colony development at the time that the control strain reached the plate boundary under each condition. Colony diameters (B) in GMM agar plates were measured every 24 h over 96 h of incubation at 37ºC. Mean and standard deviation (SD) of colonies derived from three independent mycelia cultures are represented. Asterisks indicate statistical differences in comparison with control strain (Ctrl) using two-way ANOVA with Tukey’s test for multiple comparison; ****p < 0.001.
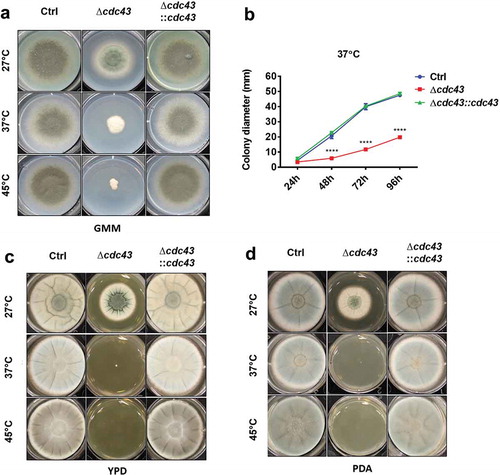
Figure 2. Lack of GGTase-I impairs A. fumigatus establishment of polarized growth.
Conidia (105) obtained from control (Ctrl), Δcdc43 or complemented (Δcdc43:cdc43) strains were inoculated on sterile coverslips submerged in GMM broth (3 ml) in six-well plates. At the designated time points, coverslips were mounted onto slides and visualized by bright field microscopy. Micromorphology of germlings at 30ºC (A) and 37ºC (B) were evaluated after 12 h, 15 h, and 24 h (scale bar = 50 µm). Germ tube formation rates at 30ºC (C) and 37ºC (D) were determined by examining at least 100 conidia. Mean and standard deviation (SD) of five independent experiments are represented, and asterisks indicate statistical differences in comparison with control strain (Ctrl) using two-way ANOVA with Tukey’s test for multiple comparison; **p < 0.01, ***p < 0.001 and ****p < 0.0001.
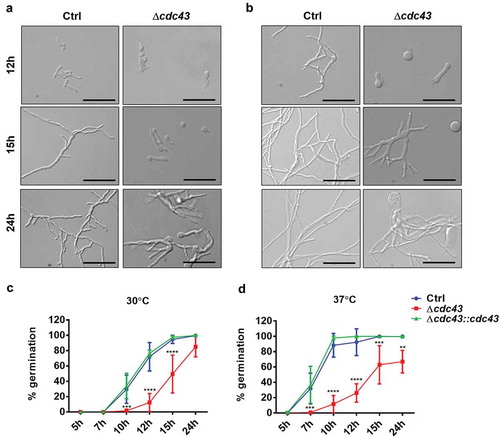
Figure 3. Loss of cdc43 alters response to cell wall disrupting compounds.
(A) Conidia (105 to 102) obtained from control (Ctrl), Δcdc43 or complemented (Δcdc43:cdc43) strains (5 µl) were spot inoculated onto GMM agar plates impregnated with Congo Red (CR) or Calcofluor White (CFW). Colony size was examined after 48 h at 37ºC. (B) Micrographs of hyphae grown for 48 h at 37ºC in GMM broth with Nikkomycin Z (scale bar = 50 µm). Samples incubated in media with no compound (NT) were used as a control.
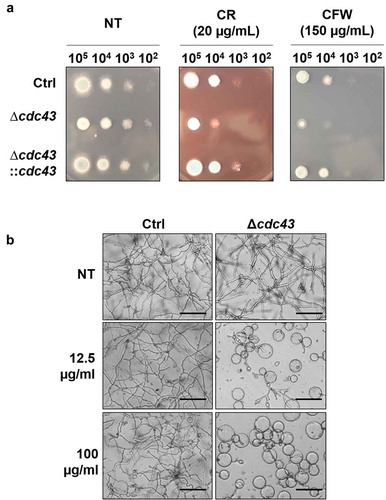
Figure 4. Protein geranylgeranylation is required for cell wall β-glucan composition.
(A) The small GTPase, RhoA, is predicted to be prenylated by the GGTase-I complex (composed of the Cdc43-RamB heterodimer) based on analysis of CAAX motif composition [Citation33]. Geranylgeranylation drives RhoA to cellular membranes where it functions as a regulatory subunit of the β-glucan synthase, FksA, complex [Citation59] and activates downstream signaling cascades (via modulation of PkcA activity) to orchestrate cell wall stress responses [Citation63]. (B) Conidia obtained from control (Ctrl) and Δcdc43 strains were inoculated in GMM broth and incubated at 30ºC or 37ºC at 250rpm. At designated time points, germlings were harvested and β-glucan staining was performed using a recombinant Fc-hDectin (10µg/ml) and anti-IgG-FITC (1:200). β-glucan staining of germlings was visualized by fluorescence microscopy (scale bar = 10 µm). Closed arrowheads denote the original germinating conidium and open arrowheads denote areas of patchy staining in hyphae of the Δcdc43 strain. Quantification of total β-glucan (C) and chitin (D) was performed after 18 h of incubation in GMM broth at 27°C, 30ºC or 37ºC with shaking at 250 rpm. Mean and standard deviation (SD) of samples derived from three independent mycelial cultures are represented, and asterisks indicate statistical differences in comparison with control strain (Ctrl) at the same temperature using unpaired t-test; *p < 0.05 and ****p < 0.0001.
![Figure 4. Protein geranylgeranylation is required for cell wall β-glucan composition.(A) The small GTPase, RhoA, is predicted to be prenylated by the GGTase-I complex (composed of the Cdc43-RamB heterodimer) based on analysis of CAAX motif composition [Citation33]. Geranylgeranylation drives RhoA to cellular membranes where it functions as a regulatory subunit of the β-glucan synthase, FksA, complex [Citation59] and activates downstream signaling cascades (via modulation of PkcA activity) to orchestrate cell wall stress responses [Citation63]. (B) Conidia obtained from control (Ctrl) and Δcdc43 strains were inoculated in GMM broth and incubated at 30ºC or 37ºC at 250rpm. At designated time points, germlings were harvested and β-glucan staining was performed using a recombinant Fc-hDectin (10µg/ml) and anti-IgG-FITC (1:200). β-glucan staining of germlings was visualized by fluorescence microscopy (scale bar = 10 µm). Closed arrowheads denote the original germinating conidium and open arrowheads denote areas of patchy staining in hyphae of the Δcdc43 strain. Quantification of total β-glucan (C) and chitin (D) was performed after 18 h of incubation in GMM broth at 27°C, 30ºC or 37ºC with shaking at 250 rpm. Mean and standard deviation (SD) of samples derived from three independent mycelial cultures are represented, and asterisks indicate statistical differences in comparison with control strain (Ctrl) at the same temperature using unpaired t-test; *p < 0.05 and ****p < 0.0001.](/cms/asset/857429b7-be35-4a1f-97f0-32ebf15fa273/kvir_a_1620063_f0004_oc.jpg)
Figure 5. The Δcdc43 mutant retains virulence during experimental invasive aspergillosis.
Neutropenic (A, B) and non-neutropenic (C, D) CF-1 female mice were intranasally inoculated with 105 conidia obtained from control (Ctrl), Δcdc43 or complemented (Δcdc43:cdc43) strains. Survival was monitored for two weeks (A, C) and analyzed using a Log-rank (Mantel-Cox) test. Histology sections of lung tissue stained with hematoxylin and eosin (H&E) and Gomori methenamine silver (GMS) collected 3 days after infection (scale bars = 100 µm) (B, D).
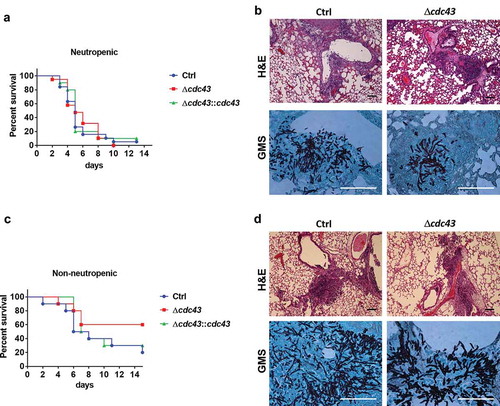
Figure 6. Protein prenylation is essential for A. fumigatus viability and virulence.
(A) Schematic representation of pTetOn promoter replacement for ramB. The pTetOn cassette was previously described and contains the pyrithiamine (ptrA) resistance marker [Citation39]. Arrows indicate areas where screening primers anneal to the genomic template. (B) Homologous integration of the pTetOn cassette was confirmed by PCR. (C) pTetOn-ramB growth in GMM media at 37°C was dependent on the presence of doxycycline in the growth media. 104 conidia from each strain were spot inoculated onto GMM with the indicated concentrations of doxycycline and incubated at 37°C for 48 h. Numbers on each well indicate concentration (µg/ml) of doxycycline. (D) Neutropenic CF-1 female mice were intranasally inoculated with 105 conidia obtained from control (Ctrl) or the tetracycline-inducible ramB (pTetOn-ramB) strains. Survival was monitored along 14 days and analyzed using Log-rank (Mantel-Cox) test. Asterisks indicate a statistically significant difference in comparison with the control group (Ctrl); ***p < 0.001.
![Figure 6. Protein prenylation is essential for A. fumigatus viability and virulence.(A) Schematic representation of pTetOn promoter replacement for ramB. The pTetOn cassette was previously described and contains the pyrithiamine (ptrA) resistance marker [Citation39]. Arrows indicate areas where screening primers anneal to the genomic template. (B) Homologous integration of the pTetOn cassette was confirmed by PCR. (C) pTetOn-ramB growth in GMM media at 37°C was dependent on the presence of doxycycline in the growth media. 104 conidia from each strain were spot inoculated onto GMM with the indicated concentrations of doxycycline and incubated at 37°C for 48 h. Numbers on each well indicate concentration (µg/ml) of doxycycline. (D) Neutropenic CF-1 female mice were intranasally inoculated with 105 conidia obtained from control (Ctrl) or the tetracycline-inducible ramB (pTetOn-ramB) strains. Survival was monitored along 14 days and analyzed using Log-rank (Mantel-Cox) test. Asterisks indicate a statistically significant difference in comparison with the control group (Ctrl); ***p < 0.001.](/cms/asset/bd137c33-e35b-4d8f-bf98-cc9e9770a658/kvir_a_1620063_f0006_oc.jpg)
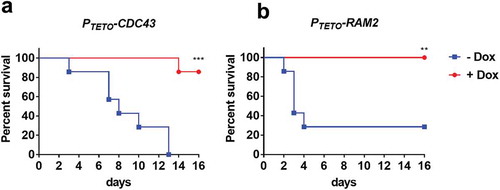
Figure 7. Both CDC43 and RAM2 are essential for Candida albicans virulence in a mouse model of disseminated infection.
Female BALB/c mice (6–8 week old) were randomly assigned into two treatment groups, one provided a gel food formulation supplemented with doxycycline (2 mg/ml) (+ Dox), the second gel food alone (- Dox). Half the mice in either group were then inoculated with 5 × 105 yeast cells of C. albicans strain PTETO-CDC43 (A), and the other half with strain PTETO-RAM2 (B) via tail vein injection (n = 7 per experimental group). Mice were monitored for 16 days, and those showing signs of distress humanely euthanized. Survival of mice in either treatment group was then compared using the Log-rank (Mantel-Cox) test, and asterisks indicate a statistically significant difference among the groups; **p < 0.01 and ***p < 0.001.