Figures & data
Table 1. Cytokine and chemokine protein levels in colon tissue from non-infected and C. rodentium-infected WT and Fpr2−/− mice at days 0, 6, and 19 PI.
Figure 1. Fpr2 cell surface localization and infection parameters in WT and Fpr2−/− mice following infection with C. rodentium. (a) Fpr2 localization at the colonic epithelial cell surface in infected (day 10, 14 and 19 PI) and non-infected mice. n = 4–9 mice/time-point. (b and c) Representative images of Fpr2 in distal colon of (b) non-infected and (c) infected WT mice at day 10 PI (arrowheads = Fpr2, L = lumen and SE = surface epithelium. Images were captured using a 40x objective. (d) Fecal CFU count of WT and Fpr2−/− mice inoculated with C. rodentium 5 × 109 CFU. (e) Spleen CFU counts of WT and Fpr2−/− mice at day 10, 14 and 19 PI. (f) Total colitis score of WT and Fpr2−/− mice at day 10, 14 and 19 PI. The score represents the sum of the following individual parameters with a maximum score of 4 for each; crypt architecture, crypt length, goblet cell depletion, number of lamina propria neutrophils, inflammatory cell infiltration, and epithelial tissue damage and ulceration. n = 4–9 mice per group. Statistics: (a) Kruskal–Wallis test followed by Dunn’s multiple comparison test, *p < 0.05, **p < 0.001 vs control, (f) Mann–Whitney U-test, #p < 0.05, crypt architecture, *p < 0.05, total colitis score WT vs Fpr2−/− at each time point. Error bars; (a) median interquartile range, D, E, and F) mean S.E.M.
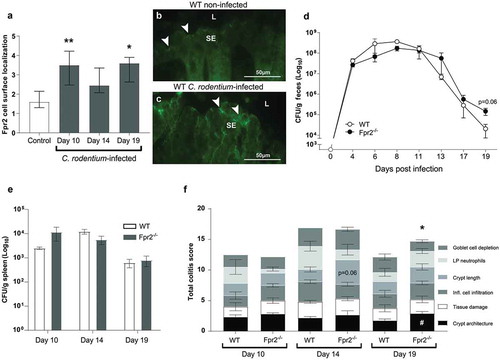
Figure 2. C. rodentium density, translocation and colitis score during early infection of WT and Fpr2−/− mice infected with a low dose of C. rodentium. (a) Fecal CFU count of Fpr2−/− mice and their WT controls inoculated with 5 × 107 CFU of C. rodentium. All Fpr2−/− mice became infected in contrast to only 6 out of 20 WT mice (n Fpr2−/− = 6, WT = 20). (b) Of the mice that became infected, the level of C. rodentium in the spleen was higher in Fpr2−/− compared to WT mice at day 6 PI and a similar trend was observed for C. rodentium in the liver (n = 6). (c) Colitis score of H&E stained distal colon sections from WT and Fpr2−/− mice at day 6 PI (n = 6, maximum score 4). Non-infected WT mice from the inoculated group (n = 14) were excluded, as they did not show signs of colitis (scores were similar to that of non-infected controls <1). (d) Manual neutrophil count for entire cross-sections of H&E stained distal colon specimens from non-infected and infected WT and Fpr2−/− mice (n non-infected = 3–4, infected = 6). (e and f) Representative H/E stained distal colon samples from infected WT (e) and Fpr2−/− (f) mice (arrows highlighting neutrophils in lamina propria). Statistics: (a–d) Mann–Whitney U-test, *p < 0.05, ##p < 0.001, and ***p < 0.0005. Error bars; (a, b, and d) Geometric mean and (c) median with interquartile range.
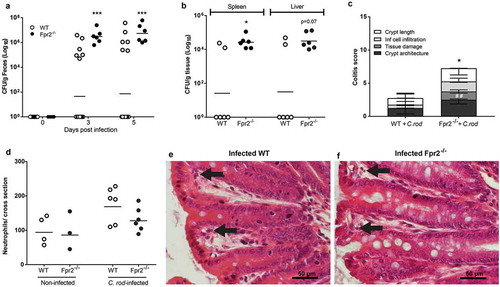
Figure 3. Mucus layer organization and bacterial localization in the distal colon of WT and Fpr2−/− mice day 6 after low dose C. rodentium infection. (a–d) Representative immunofluorescence images of MUC2 (green) and nuclei (DAPI, blue) showing the presence of an inner mucus layer with a striated appearance (indicated by ||) separating luminal contents from the epithelium in non-infected control (a) and infected WT (b) mice, respectively. This was in contrast to Fpr2−/− mice in which a clearly striated inner mucus layer was absent regardless of their infection status (c and d). (e–h) Bacterial localization (EUB338 probe hybridizing with eubacteria including C. rodentium, green) at the epithelium and crypts (outlined by Cell Mask, red) in the distal colon, white boxes show close-ups of the surface epithelium for each genotype/image. In WT non-infected control mice, the vast majority of bacteria were at a distance from the epithelial surface (e) whereas a low amount of bacteria were in contact with the epithelium in the infected WT mice (f) and Fpr2−/− non-infected control mice (g) and a large amount of bacteria were present in close contact with the epithelium in infected Fpr2−/− mice (h). (i and j) C. rodentium localization in infected WT (i) and Fpr2−/− (j) mice detected using an antisera targeting the O152 antigen (also found in C. rodentium). (k) Quantification of bacterial density in close proximity of the epithelial surface or colonic crypts in C. rodentium infected WT and Fpr2−/− mice. n = 5–6. Statistics: (k) Kruskal–Wallis test followed by Dunn’s multiple comparison test, ****p < 0.0001 vs WT non-infected control, ##p < 0.005 vs WT + C. rod. Error bars; Median interquartile range.
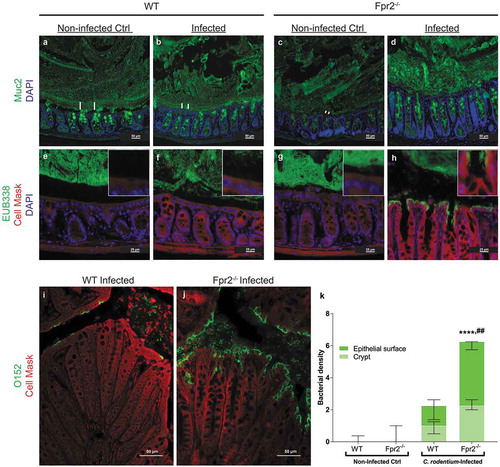
Figure 4. Mucin density in crypts and goblet cell mucin production and transport en route secretion during early C. rodentium infection. (a–d) Representative PAS/Alcian blue-stained distal colon samples from WT and Fpr2−/− mice infected with 5 × 107 CFU of C. rodentium at day 6 PI using 10x and 20x objective from infected WT (a and b) and Fpr2−/− (c and d) mice. (e) Density score of mucus along the crypts separated into; bottom-, mid- and upper-crypt. Each compartment of the crypt received a score from 0 to 3 based on density, &p < 0.05 upper crypt, #p < 0.05 mid crypt, ¤¤p < 0.005 bottom crypt, **p < 0.005 total score. n = 5–6 mice. (f) Blinded visual semi-quantification of the intensity of incorporated GalNAz in goblet cells (n = 5–6 mice). (a) Schematic representation of a goblet cell and the four compartments analyzed for GalNAz incorporation, N refers to the nucleus. (f) Each location received a score of 0–3 based on intensity, *p < 0.05 perinuclear, #p < 0.05 mid cytoplasm. Mann–Whitney U-test. Error bars; median with interquartile range.
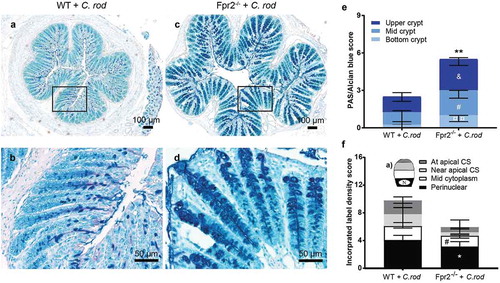
Figure 5. Mucin O-glycans from C. rodentium infected WT and Fpr2−/− mice during early colonization characterized by mass spectrometry. (a) Size distribution (number of monosaccharide residues/glycan) derived from the overall compositions of O-glycans. (b) Relative abundance (%) of O-glycans containing 1–4 sialic acid residues. (c) relative abundance (%) of sulfated structures detected on GlcNAc. (d) Relative abundance (%) of terminal residues on O-glycans. n = 3 mice. Statistics: Unpaired t-test, (a) glycan chain lengths of 3–6 pooled and 7–15 pooled. Error bars; Mean S.E.M.
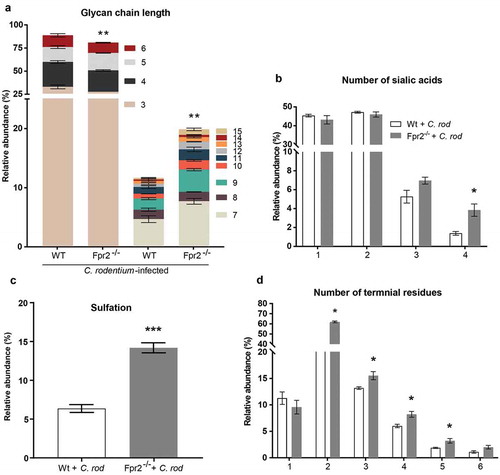
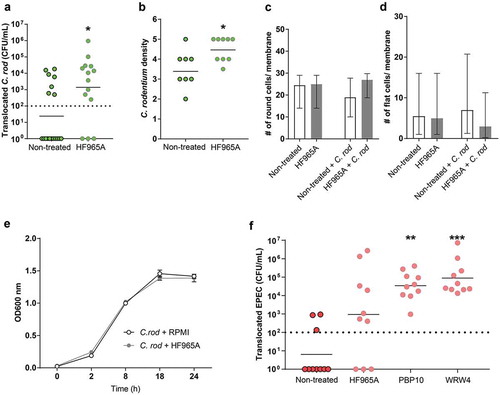
Figure 6. Effect of FPR inhibitor on human in vitro colonic mucosal surfaces during C. rodentium infection. (a) CFU in the basolateral compartment (i.e., amount of bacteria that crossed the in vitro mucosal surface) of non-treated and FPR2 inhibitor (HF965A) treated C. rodentium infected in vitro mucosal surfaces at 8 h PI (dotted line highlights minimum detection limit, n = 14–18). (b) Semi-quantification of bacterial density in C. rodentium infected non-treated and treated in vitro mucosal surfaces (n = 8–9). (c and d) The number of round (c), and flat (d) cells found along the entire in vitro mucosal surface section with/without treatment and with/without infection (n = 3–8). (e) C. rodentium growth in the presence or absence of HF965A measured for a duration of 24 h at OD 600 nm (n = 6). (f) CFU in the basolateral compartment of non-treated and FPR2 inhibitor-treated EPEC infected in vitro mucosal surfaces at 5 h PI (dotted line highlights minimum detection limit, n = 10). Statistics: (a and b) Mann–Whitney U-test, *p < 0.05 vs non-treated. (f) Kruskal–Wallis test followed by Dunn’s multiple comparison test, **p < 0.005, ***p < 0.0001 vs non-treated. Error bars; (a, b and f) geometric mean, (c and d) median interquartile range and (e) Mean S.E.M.