Figures & data
Figure 1. Overview of leucine biosynthesis in A. fumigatus. As shown in S. cerevisiae [Citation6], 2-ketoisovalerate, a common step of the biosynthesis of valine and leucine, is converted by LeuC (α-IPM synthase) to α-IPM. LeuA (α-IPM isomerase) produces β-IPM, which is subsequently converted by Leu2A (dehydrogenase), spontaneous decarboxylation and Bat2 (BCAA aminotransferase) to leucine. The biosynthesis is feedback-inhibited via blocking of LeuC enzymatic activity by the end-product leucine [Citation6]. The intermediate α-IPM was shown to posttranslationally activate LeuB in S. cerevisiae and A. nidulans [Citation6,Citation7]. The results of this study indicate that α-IPM also activates LeuB in A. fumigatus including the regulation of BCAA encoding genes, gdhA, and genes involved in adaptation to iron starvation.
![Figure 1. Overview of leucine biosynthesis in A. fumigatus. As shown in S. cerevisiae [Citation6], 2-ketoisovalerate, a common step of the biosynthesis of valine and leucine, is converted by LeuC (α-IPM synthase) to α-IPM. LeuA (α-IPM isomerase) produces β-IPM, which is subsequently converted by Leu2A (dehydrogenase), spontaneous decarboxylation and Bat2 (BCAA aminotransferase) to leucine. The biosynthesis is feedback-inhibited via blocking of LeuC enzymatic activity by the end-product leucine [Citation6]. The intermediate α-IPM was shown to posttranslationally activate LeuB in S. cerevisiae and A. nidulans [Citation6,Citation7]. The results of this study indicate that α-IPM also activates LeuB in A. fumigatus including the regulation of BCAA encoding genes, gdhA, and genes involved in adaptation to iron starvation.](/cms/asset/6e0c122e-d995-4a5e-a367-805b5c705baf/kvir_a_1682760_f0001_oc.jpg)
Figure 2. Strains lacking LeuA or LeuC display leucine auxotrophy and ∆leuC requires higher leucine supplementation for growth. Growth of ∆leuA and ∆leuC compared to wt and the reconstituted strains was analyzed on minimal medium (a) complete medium (b) or 25% blood agar (c). Generally, 103 spores were point-inoculated on the respective solid growth medium and incubated at 37°C. Minimal medium contained different concentrations of ferrous iron (0 mM, 0.03 mM, or 4 mM), the ferrous iron-specific chelator BPS, and/or leucine (0 mM, 1 mM, 2 mM, 5 mM, or 9 mM) as indicated. 0.03 mM iron reflects the standard minimal medium. Growth was evaluated after incubation for 48 h on minimal medium, 24 h on complete medium and 36 h on blood agar. Representatives of at least three biological replicates are shown.
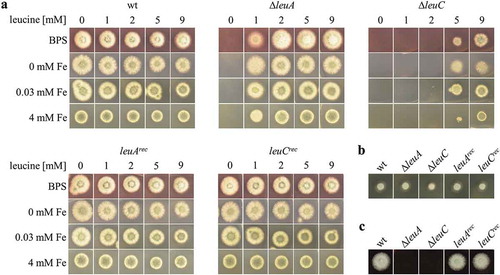
Figure 3. Lack of LeuC results in decreased growth and TAFC production in iron-limited liquid cultures. 106 spores/mL of the respective strains were cultured in 100 mL liquid minimal medium containing 0, 1, 2, 5 or 9 mM of leucine with or without addition of 0.03 mM of FeSO4 at 37°C for 30 h. (a) For the wt, the absolute biomass is displayed, while for the deletion and reconstituted strains, the biomass is normalized to the wt biomass under the same conditions. (b) TAFC content of the culture supernatants was normalized to the respective biomass. TAFC production of the wt is shown normalized to that without leucine supplementation, while TAFC production of the deletion and reconstituted mutant strains is shown normalized to the wt production at the same leucine concentration. The data show the mean ± standard deviation of three biological replicates.
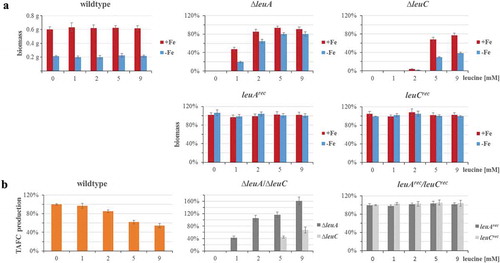
Figure 4. Transcript levels of leuB and LeuB target genes are upregulated in ∆leuA but downregulated in ∆leuC compared to wt (a) and downregulated by leucine supplementation in wt (b). (a) For Northern blot analysis, fungal strains were cultured under iron starvation conditions with 5 mM leucine supplementation. The wt strain was grown for 16 h, ∆leuA for 20 h, and ∆leuC for 28 h to compensate for the different growth rate and to reach the same biomass. (b) To analyze potential autoregulation of LeuB, the wt strain was cultured for 16 h under iron starvation prior the addition of leucine to a final concentration of 5 mM. For Northern analysis, samples were taken at 0, 15 and 30 min after leucine addition.
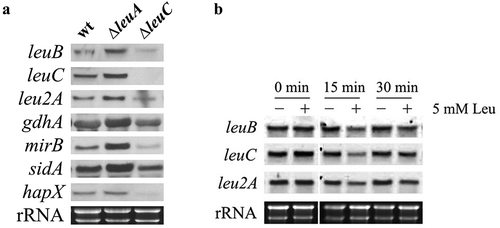
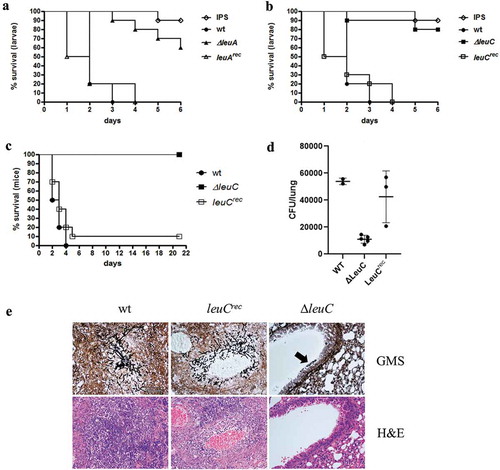
Figure 5. ∆leuA and ∆leuC mutants display attenuated virulence in Galleria mellonella and ∆leuC is avirulent in a pulmonary aspergillosis mouse model. In G. mellonella, ∆leuA (a) and ∆leuC (b) showed significantly increased survival rates compared to wt (Log-Rank test, p < 0.0001), while survival of the reconstituted strains (leuArec and leuCrec) was statistically indifferent to the wt (p = 0.80 and 0.56, respectively). The curves display the average survival of three independent experiments (in total 60 larvae per sample). (c) In the mouse model (10 mice per strain), the leuC deletion strain was avirulent compared to the wt and the reconstituted strain (p < 0.0001). (d) Mouse lung fungal burden is greatly reduced in mice infected with the LeuC-deletion strain compared to the wt (p < 0.0001) and reconstituted strain (p < 0.008). (e) Histological analysis of infected lungs stained with GMS (gomori methenamine silver, stains fungi black, upper panel) shows large foci containing numerous invasive hyphae in mice infected with the wt and reconstituted strain. In contrast, only small infrequent aggregates of swollen conidia were seen in lung sections of mice infected with the ∆leuC strain (black arrow, upper panel on the right). Staining of lung cells with H&E shows granulocyte accumulation around the wt and leuCrec A. fumigatus strains, but not the ∆leuC strain (lower panel).