Figures & data
Figure 1. Biosynthesis and signaling system of pyocyanin [Citation15]. Chorismic acid is transformed into phenazine-1-carboxylic acid by the PhzA to G proteins. Then, phenazine-1-carboxylic acid is subsequently converted into different phenazines by the enzymes PhzH, PhzS, and PhzM, respectively. The product of the 5-methylphenazine-carboxylic acid betaine is further transformed into pyocyanin (PYO) by PhzS
![Figure 1. Biosynthesis and signaling system of pyocyanin [Citation15]. Chorismic acid is transformed into phenazine-1-carboxylic acid by the PhzA to G proteins. Then, phenazine-1-carboxylic acid is subsequently converted into different phenazines by the enzymes PhzH, PhzS, and PhzM, respectively. The product of the 5-methylphenazine-carboxylic acid betaine is further transformed into pyocyanin (PYO) by PhzS](/cms/asset/b8db6a41-6188-4136-8a7d-134a95f67523/kvir_a_1708052_f0001_oc.jpg)
Figure 2. Model of the P. aeruginosa quorum-sensing hierarchy. When cells reach a threshold density, the las quorum sensing will be induced. LasI directs the synthesis of 3-oxo-C12-HSL, which then binds to and activates LasR. LasR regulates the production of PQS, which is conversed by PqsH from HHQ, catalyzed by PqsA-E. PQS either directly or indirectly induces rhlI, which leads to the production of C4-HSL that binds to and activates RhlR. Hence, PQS constitutes a regulatory link between the las and rhl quorum-sensing system. PQS binds to the transcriptional regulator PqsR to regulate biofilm formation and virulence factor production. The RhlR–C4-HSL complex can induce genes controlled by the rhl quorum-sensing system, such as biofilm formation and virulence factor production. 3-oxo-C12-HSL has an inhibitory effect on the association between RhlR and C4-HSL
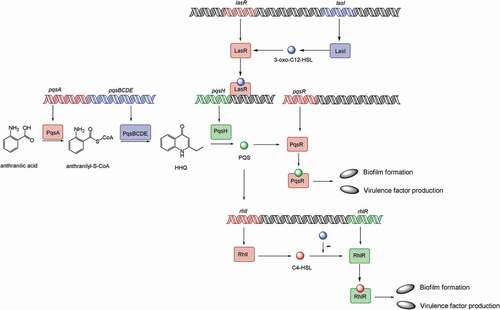
Table 1. Quantitation of pyocyanin in P. aeruginosa PAO1 and the knockout mutants
Figure 3. Effect of mvaT mvaU knockouts on the phenotype of P. aeruginosa PAO1. A: Percent survival of mice infected by PAO1 wild type and mvaT mvaU knockout mutants (n = 10), ***P< 0.001 via log-rank test. PAO1: 6.5 × 103 CFU/mice; PAO1ΔT: 4 × 103 CFU/mice; PAO1ΔU: 6.5 × 103 CFU/mice; PAO1ΔTΔU:1.5 × 104 CFU/mice. B: Biofilm formation of PAO1 wild type and mvaT mvaU knockout mutants, calculated with one-way ANOVA and Bonferroni’s multiple comparisons (n = 6), *P< 0.05, ***P< 0.001, ****P< 0.0001. C: Relative growth of the mvaT mvaU knockout mutants in comparison to PAO1, calculated with one-way ANOVA and Bonferroni’s multiple comparisons (n = 4), ***P< 0.001
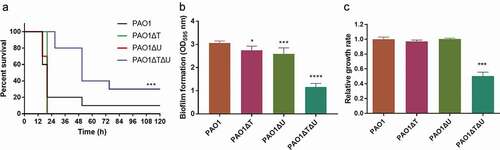
Figure 4. Effect of mvaT mvaU knockout mutations on gene expression of pyocyanin biosynthesis system. A: β-galactosidase activity of phzA-G1/A2-G2/H/M/S: lacZ fusion covering the regulatory region. B: Relative gene expression of pyocyanin biosynthesis genes detected by Real-Time PCR. Data were calculated with one-way ANOVA and Bonferroni’s multiple comparisons, in comparison to P. aeruginosa PAO1, *P< 0.05, **P< 0.01, ***P< 0.001, ****P< 0.0001
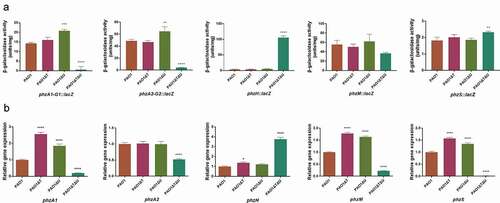
Figure 5. Effect of mvaT mvaU knockout mutations on the expression of genes involved in QS system determined by Real-Time PCR. A: Relative expressions of genes coding QS signal molecule synthetase. B: Relative expressions of genes coding transcriptional activator proteins binding with signal molecules. Data were calculated with one-way ANOVA and Bonferroni’s multiple comparisons, *P< 0.05, **P< 0.01, ***P< 0.001, ****P< 0.0001
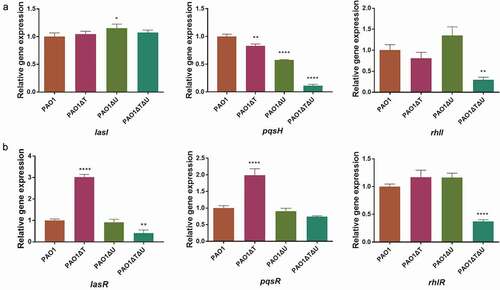
Figure 6. Effect of mvaT mvaU knockout mutations on the production of QS system signal molecules AHLs (3-oxo-C12-HSL and C4-HSL) and PQS determined by LC-MS/MS. A: Quantitation of 3-oxo-C12-HSL. B: Quantitation of PQS. C: Quantitation of C4-HSL. Data were calculated with one-way ANOVA and Bonferroni’s multiple comparisons, *P< 0.05, **P< 0.01, ***P< 0.001, ****P< 0.0001
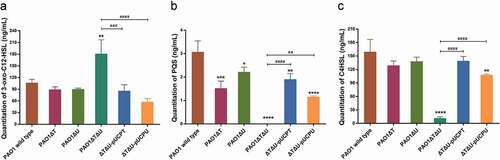
Table 2. List of selected genes whose transcriptions were affected by mvaT mvaU mutations
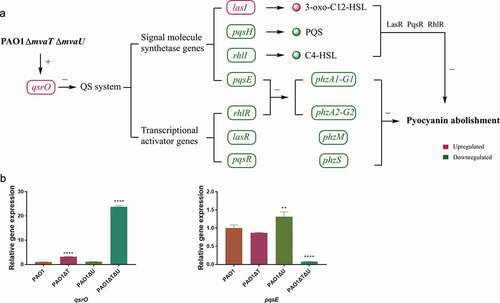
Figure 7. Interactions of mvaT and mvaU to control biosynthesis and signaling systems of pyocyanin. A: When mvaT and mvaU were knockout, expression of qsrO was significantly increased, which led to the decreased expression of genes coding signal molecule synthetases (pqsH, rhlI, and pqsE) and transcriptional activators (rhlR, lasR, and pqsR). The lower expression levels of phzA1-G1 and phzA2-G2 caused by decreased expression of rhlR and pqsE, together with lower expression levels of phzM, phzS and the decreased formation of signal-transcriptional activator complexes resulted in pyocyanin abolishment. B: Relative gene expressions of qsrO and pqsE detected by Real-Time PCR. Data were calculated with one-way ANOVA and Bonferroni’s multiple comparisons, in comparison to P. aeruginosa PAO1, **P< 0.01, ****P< 0.0001