Figures & data
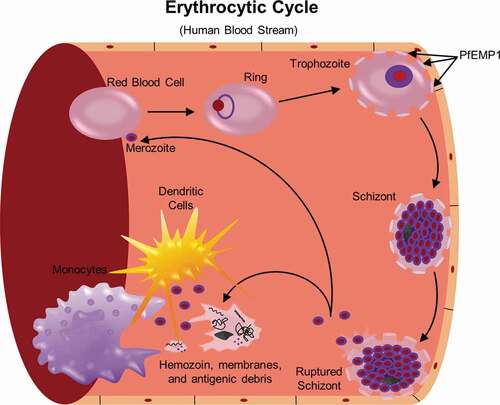
Figure 1. Life cycle of P. falciparum. Infection of the human host occurs when a female Anopheles mosquito bites and injects P. falcipraum sporozoites from their salivary glands into ahost capillary during a blood meal. Sporozoites that enter the bloodstream travel to the liver and invade hepatocytes. Over the course of 7 days, a single sporozoite undergoes asexual reproduction within a hepatocyte to produce ~40,000 merozoites that are released into the bloodstream when the hepatocyte ruptures. The released merozoites invade erythrocytes, beginning the 48 hr erythrocytic life cycle as ring stage parasites. During maturation to a trophozoite, the parasites modify the erythrocyte surface by forming knobs containing PfEMP1 proteins that adhere to the microvasculature and prevent parasite clearance by the spleen. The parasite remains sequestered as it undergoes 4–5 rounds of asexual reproduction, producing a schizont containing 16–32 merozoites that are released during schizont rupture along with hemozoin, membranes, and antigenic debris that can stimulate early innate immunity. A subset of intraerythrocytic parasites undergo sexual differentiation and develop for 10–12 days within the bone marrow into either a male or a female gametocyte. Mature stage V gametocytes re-enter the circulation and can be taken up by a female mosquito to propagate the infection cycle. Within the mosquito midgut, these male and female gametocytes are stimulated immediately to form microgametes and macrogametes, respectively, which fertilize. Over the next 24 hr, the zygote develops into an ookinete, migrates across the midgut epithelium and becomes an oocyst that in 2–3 weeks can produce thousands of sporozoites. The sporozoites are released upon oocyst rupture and migrate to the mosquito salivary glands, ready to begin the cycle in a new human host
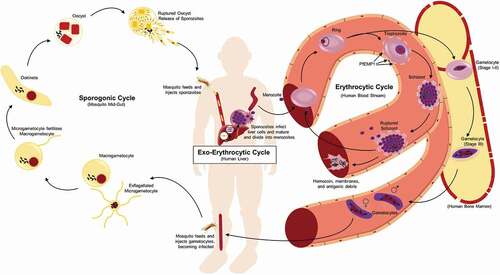
Figure 2. Neutrophils engage in the innate immune response through phagocytosis and production of reactive oxygen species (ROS) and neutrophil extracellular traps (NETs). Following schizont rupture, antigenic debris are released from the iRBC, including heme and digestive vacuoles that contain hemozoin. Through their interaction with complement, neutrophils are able to phagocytose hemozoin-containing digestive vacuoles [Citation82]. ROS occurs within this phagolysosome, a process by which oxygen is converted to superoxide by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX), and then superoxide is converted into H2O2 and hydroxyl radicals (•OH). NOX can be present on the plasma membrane and phagosomal membrane, and is capable of diffusion outside of the neutrophil, as well [Citation148]. Heme, a byproduct of RBC rupture during schizont release [Citation88], can also trigger the release of NETs, structures composed of extracellular deoxyribonucleic acid (DNA), chromatin, and granule proteins that can ensnare and kill pathogens [Citation80]
![Figure 2. Neutrophils engage in the innate immune response through phagocytosis and production of reactive oxygen species (ROS) and neutrophil extracellular traps (NETs). Following schizont rupture, antigenic debris are released from the iRBC, including heme and digestive vacuoles that contain hemozoin. Through their interaction with complement, neutrophils are able to phagocytose hemozoin-containing digestive vacuoles [Citation82]. ROS occurs within this phagolysosome, a process by which oxygen is converted to superoxide by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX), and then superoxide is converted into H2O2 and hydroxyl radicals (•OH). NOX can be present on the plasma membrane and phagosomal membrane, and is capable of diffusion outside of the neutrophil, as well [Citation148]. Heme, a byproduct of RBC rupture during schizont release [Citation88], can also trigger the release of NETs, structures composed of extracellular deoxyribonucleic acid (DNA), chromatin, and granule proteins that can ensnare and kill pathogens [Citation80]](/cms/asset/b8d95e8e-b175-4bb5-95ae-1b48517a1ef4/kvir_a_1708053_f0002_oc.jpg)
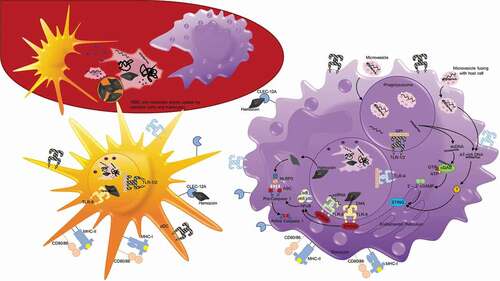
Figure 3. Antigen uptake and recognition of P. falciparum during the erythrocytic cycle. During each asexual cycle, schizont rupture releases merozoites as well as membrane fragments, DNA, RNA, and hemozoin that can be recognized and taken up by white blood cells, including dendritic cells and monocytes depicted here. Plasmodium antigens may also enter host cells, including monocytes, in the form of microvesicles that are phagocytosed or fuse with host cells. On their surface, monocytes and dendritic cells possess toll-like receptors including TLR1/2 and TLR4 that recognize glycophosphatidylinositol (GPI). They also possess C-type lectin receptor (CLEC) 12A that can specifically recognize extracellular hemozoin crystals. Additionally, after merozoites or ruptured parasite debris is phagocytosed, protein antigens can be degraded and presented on MHC molecules on the cell surface to activate T cells. Within the phagolysosomes, TLR8 can recognize ssRNA and TLR9 can recognize DNA that binds to hemozoin. TLR8 and TLR9 both signal through myeloid differentiation primary response protein 88 (MYD88), which activates nuclear factor-κB (NF-κB), and induces the production of proinflammatory cytokines via the activation of caspase 1. Hemozoin crystals can also destabilize the phagolysosomes, allowing entry into the host cytoplasm and activation of NOD-, LRR- and pyrin-domain-containing 3 (NLRP3) that assembles into inflammasomes. In the cytoplasm, NLRP3 combines with adaptor protein ASC to form Pro-caspase 1, and when activated, inflammasomes activate caspase 1 and cleave and secrete interleukin-1β (IL-1β). dsDNA that enters the cytoplasm with hemozoin or enters directly by microvesicle fusion to the plasma membrane can activate two additional signaling pathways. dsDNA can bind cyclic GMP-AMP synthase (cGAS), stimulating the generation of the second messenger cyclic GMP-AMP (cGAMP). 2ʹ-3ʹ cGAMP binds and activates stimulator of IFN genes protein (STING) in the endoplasmic reticulum that activates serine/threonine-protein kinase TBK1, leading to the production of type 1 interferons. AT-rich DNA in the cytoplasm can activate an unknown receptor that converges on this same STING–TBK1–IRF3 pathway