Figures & data
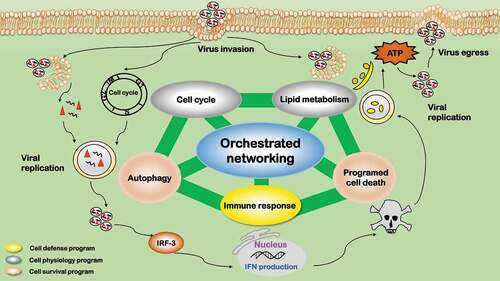
Figure 1. Pro-viral roles of autophagy during virus infection. Autophagy facilitates viral replication in six aspects. 1) Providing delivery vehicle. Viruses hijack host autophagy pathway to deliver viral particles to the replication sites. 2) Offering replication platform. Autophagosomes provide the scaffold and concentrate nutrients for viral replication, and protect the intermediates from immune detection. 3) Providing nutrients. Autophagy provides nutrients for viral replication. 4) Availing maturation and egress. Autophagy machinery facilitates in the late stage of viral lifecycle including capsid maturation, envelopment (if applicable), and egress. 5) Avoiding immune surveillance. Viruses modulate autophagy signaling by inhibiting MHC antigen presentation to avoid immune surveillance and create a favorable environment for viral survival. 6) Avoiding programmed cell death. Viruses have developed strategies to suppress autophagy and avoid programmed cell death. The background depicts key components of the autophagy pathway. The major roles played by autophagy are summarized in the middle, with the relevant components of each role listed close by
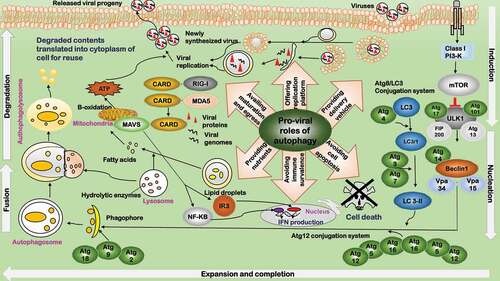
Figure 2. Pro-viral roles of programmed cell death during virus infection. The pro-viral roles related to programmed cell death include: 1) Preventing cell death. Cell death is inhibited during early viral invasion to provide a cellular environment for viruses to replicate. 2) Suppressing immune response. By adopting a tolerogenic type of cell death, programmed cell death paralyzes host’s innate and adaptive defense systems to foster a favorable environment for the progression of viruses and their progenies. 3) Availing viral egress. By subverting host’s immune response, programmed cell death avails viral egress
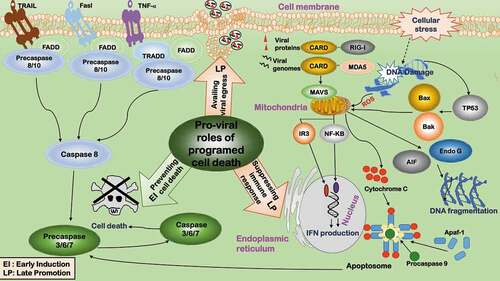
Figure 3. Pro-viral roles of immune suppression during virus infection. Immune suppression favors viral infection in two aspects. 1) Clearing infection. 2) Halting the process till adaptive response. The innate immune response is interconnected with and fed into the scheme of adaptive response via interferons (IFNs). Viruses inhibit immune response by either suppressing IFN induction or IFN response genes. Viruses suppress IFN induction in four ways. ①Escaping immune surveillance. Viruses have evolved various strategies to escape innate immune surveillance. ②Inhibiting cellular sensors. Some viruses target cellular sensors of the immune response such as RIG-I to suppress IFN response. ③Inhibiting IFN-regulatory factors. Some viruses inhibit immune response by targeting IFN-regulatory factors such as IRF3. Viruses suppress IFN response genes, e.g. ①inhibiting PKR activity, ②blocking RNase L activation, ③degrading PML nuclear bodies, and ④suppressing cellular restriction factors
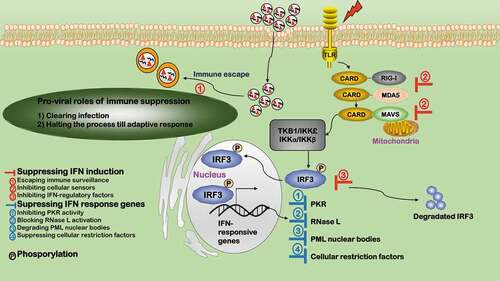
Figure 4. Pro-viral roles of cell cycle alteration during virus infection. Three types of cell cycle arrests exist to favor viral replication in response to DNA damage or other environmental changes for the sake of accurate genetic material passaging, i.e. G2/M (red), G1/S (purple), G0/G1 (blue). Cell cycle arrest plays four pro-viral roles. 1) Reducing immune response. 2) Preventing cell early death. 3) Modulating the expression of viral/cellular genes critical for their life cycle completion. 4) Pushing cells to the phase that provides optimal conditions for virus replication. Cell cycle arrest can be caused by 1) incapable of passing through the checkpoint and entering the next phase or 2) being attracted in or not capable of exiting the current phase. Viruses have developed three strategies to make cells “incapable of passing through the checkpoint”: ①Inhibiting CDK. Some viruses produce proteins to activate CDK inhibitors. ②Inhibiting cyclin. Some viral proteins interact with cyclins. ③Inhibiting CDK/cyclin complex. Some viruses block nuclear transportation or accumulation of CDK-cyclin complexes. Cell cycle promotion (green) typically pushes cells toward the S phase that provides optimal conditions for virus replication through inhibiting pRb and TP53 signalings
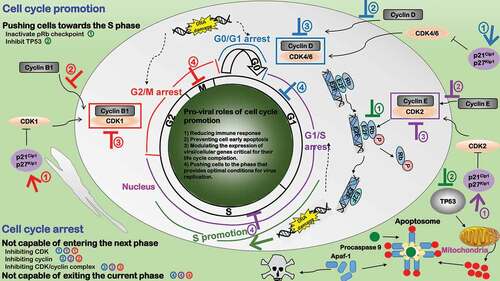
Figure 5. Pro-viral roles of lipid metabolic reprogramming during virus infection. Lipids have four pro-viral roles. 1) Virus entry and trafficking. Lipids can serve as the attachment factor, internalization receptor, or transportation shuttle during viral entry. 2) Virus replication and assembly. Lipids can offer subcellular space for key events to occur in the viral life cycle. 3) Energy and nutrient production. Lipids are required for viral replication as a source of energy and nutrients. 4) Virus egress. Lipids are important components for viral envelopment and play various roles during viral egress
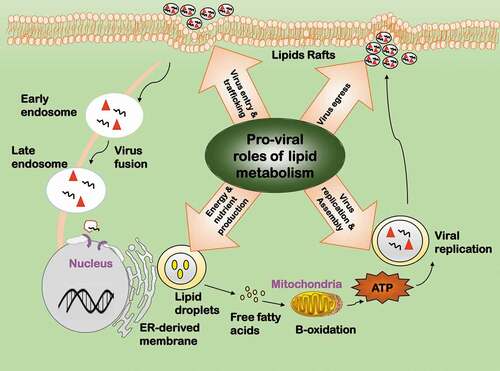
Figure 6. Orchestrated networking among the five influential processes during virus infection. The five influential factors form an orchestrated network governing the fate of viruses after virus infection. Autophagy and programmed cell death are cell survival programs, which are oppositely switched on/off at different stages by viruses to create a favorable environment for virus survival. Immune response is a cell defense program. Cell cycle alteration and lipid metabolic reprogramming are adaptations to cell housekeeping physiologies. Cell defense and physiology adaptations orchestrate and dynamically tilt cells between the two cell survival programs (autophagy and programmed cell death) on virus infection