Figures & data
Table 1. Insect bioassays against the wax moth larvae
Figure 1. Schematic structure and expression analysis of the metalloprotease MrM35-4. (a) Schematic structuring of the enzyme. The conserved domain and motif are indicated. (b) Transcription of MrM35-4 by the fungus at different stages. RNA was extracted from different samples including: CO, conidia harvested from 2-week-old PDA plate; MY, mycelia harvested from 3-day-old SDB culture; AP, appressoria induced on locust wings for 36 h; HB, hyphal-body cells harvested from the hemocoel of wax moth larvae 48 h post-injection with fungal spores. (c) Protein expression and secretion analysis. The WT spores were inoculated in SDB or MM medium containing 1% silkworm pupa homogenate for 3 days, and the proteins concentrated from the supernatants were used for western blot (WB) analysis with the polyclonal antibody raised against MrM35-4. The uninoculated SDB and 1% cuticle samples were included as mock controls. (d) In vivo detection of the enzyme in insects after injection with fungal spores for different times. The monoclonal antibody raised against the GmAct3 of G. mellonella was used in WB analysis as a reference
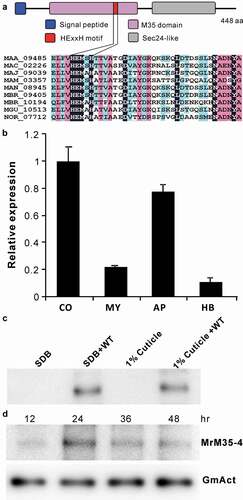
Figure 2. Insect survivals. (a) Survival of the wax moth larvae after topical infection with the WT and mutant strains. (b) Survival of the wax moth larvae after injection with the spores of the WT and mutant strains. The last instar larvae of wax moth were used for bioassays. (c) Survival of the wild-type D. melanogaster after topical infection with the WT and different mutants of M. robertsii.
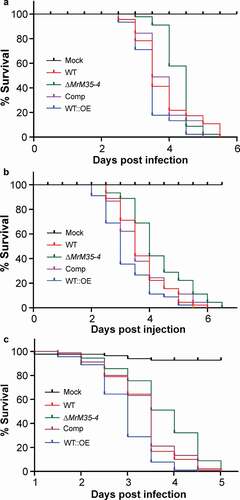
Figure 3. Comparative proteolytic and penetration assays between WT and mutant strains. (a) Casein degradation. Spore suspension (3 μl of 1 × 106 conidia ml−1 each spot) was inoculated on MM medium containing 1% casein for 3 days. (b) Caseinolytic assay with the purified protease. Different amounts of MrM35-4 were loaded in MM medium containing 1% casein for 4 h. (c) Cellophane membrane penetration assays. The phenotypes of the WT and mutants grown on the MM medium after the inoculated cellophane membranes were removed for 4 days. (d) Locust wing penetration assays. The locust hind wings lined on the MM medium were inoculated in the middle for 3 days (top panels) and then removed. The plates were kept for incubation for another 4 days (lower panels). (e, f) Comparative analysis of the colony diameter size after removing the cellophane membranes (e) or locust hind wings (f) for 4 days. The difference was compared between WT and individual mutant. **, P < 0.01
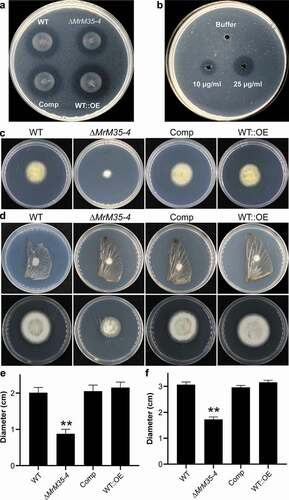
Figure 4. Suppression of insect immune responses. (a) Cuticular melanization of wax moth larvae. The insects were immersed in the spore suspension of each strain for 30 sec and photographed 48 h post-inoculation. (b) Hemocyte encapsulation and melanization. The wax moth larvae were injected with the spore suspensions of each strains and individual insects were bled at different time post-injection to observe insect immune responses against different strains. Fungal cells escaped from hemocyte attack are arrowed. (c) Expression of the antifungal galllerimycin (Gal) gene in wax moth larvae after infection with WT and mutant strains. The wax moth larvae injected with fungal spores for 36 h and the fat bodies were isolated for RNA extraction to determine gene expression. (d) Expression of the antifungal drosomycin (Drs) gene in Drosophila after infection with the WT and mutant strains of M. robertsii. The female adults of Drosophila were infected and used for RNA extraction 36 h post-topical infection
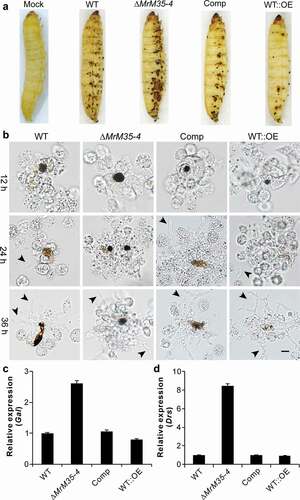
Figure 5. Activity inhibition and degradation of PPOs by MrM35-4. Deactivation of rPPO1 (a) and rPPO2 (b) activity. The reaction buffer (50 μl) contains 50 μg of rPPO1 and 1 μg of either MrM35-4 or α-chymotrypsin (α-Chy) and the tubes were photographed after reaction for 15 min. Comparative degradation of rPPO1 (c) and rPPO2 (d) by MrM35-4 and α-Chy for different times. Arrowed bands show the differences between two enzyme treatments
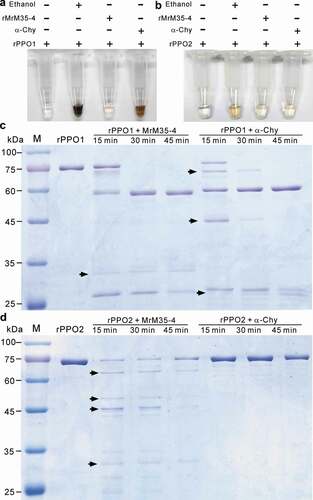
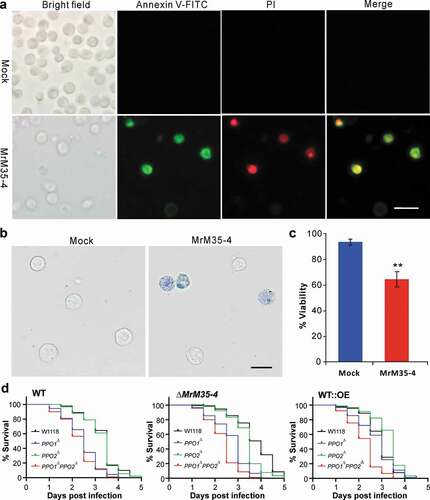
Figure 6. Induction of cell apoptosis and bioassays against different fly strains. (a) Annexin V-FITC staining assay. The S2 cells were incubated with or without MrM35-4 for 1 h and then harvested in PBS buffer by staining with the FITC-conjugated annexin V and PI (propidium iodide) for 30 min in dark. The dying cells could bind annexin V-FITC showing green staining, and those cells that lost membrane integrity could be stained by PI in red. (b) Trypan blue staining. The S2 cells were incubated with or without MrM35-4 for 3 h and then stained with trypan blue for 10 min. (c) Comparison of apoptotic cells between mock control and MrM35-4 treatments. Apoptotic cells were counted after trypan blue staining. **, t-test, P = 0.0011. (d) Survival of the wild-type, PPO1∆, PPO2∆, and PPO1∆PPO2∆ mutants of D. melanogaster after topical infection with the WT and different mutants of M. robertsii.