Figures & data
Table 1. Primers used in this study.
Figure 1. A schematic diagram for the construction of Vero/TMPRSS2 and Vero/MSPL cell lines, which were generated following the steps of arrows. (a) The constitutive recombinant plasmids (lentiviral vector) FUGW-TMPRSS2 and FUGW-MSPL containing TMPRSS2 and Vero/MSPL genes, (b) lentivirus envelope plasmids pMD2.G and psPAX2, (c) pseudotyped lentivirus particles produced in the HEK293 T cells, transduced to the Vero cells, and established in the cell lines by screening of the fluorescence expression.
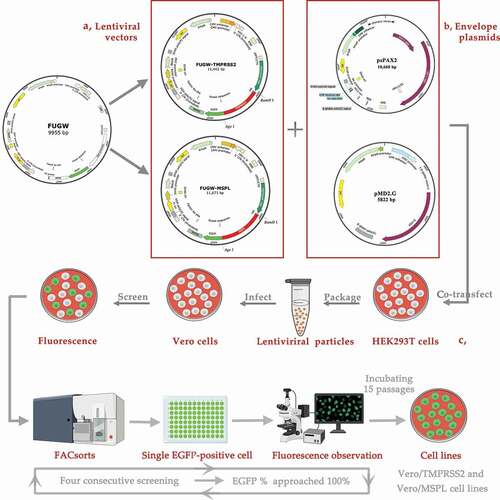
Figure 2. The expression of TMPRSS2 and MSPL genes in the HEK293 T cells. (a) The expression of TMPRSS2 and MSPL was detected by western blot with anti-HA monoclonal antibody after 72 h post-transfection. Immunoblot of TMPRSS2 and MSPL with the expected size was developed, but not for FUGW. FUGW: lentivirus FUGW in HEK293 T cells; TMPRSS2: lentivirus FUGW-TMPRSS2 in HEK293 T cells; MSPL: lentivirus FUGW-MSPL in HEK293 T cells. (b and c) TMPRSS2 and MSPL genes were detected by reverse transcription PCR. M: DNA Marker; N: negative control (water as negative control); FUGW: FUGW control plasmid without target genes. (d) Fluorescence images of FUGW/HEK293 T cells, TMPRSS2/HEK293 T cells, and MSPL/HEK293 T cells were observed after 72 h post-transfection.
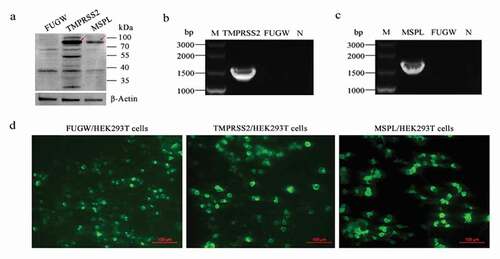
Figure 3. Stable expression of TMPRSS2 and MSPL genes in Vero cell lines. (a) The proteins in the lysates of Vero/TMPRSS2 and Vero/MSPL cell lines after consecutively incubating 15 passages were separated by SDS-PAGE, followed by identification by western blot with anti-HA monoclonal antibody. FUGW: FUGW/Vero cell lines; TMPRSS2: Vero/TMPRSS2 cell lines; MSPL: Vero/MSPL cell lines. (b) TMPRSS2 and MSPL genes were detected in Vero/TMPRSS2 and Vero/MSPL cell lines after consecutively incubating 15 passages by reverse transcription PCR. M: DNA Marker; N: negative control (water as negative control); FUGW: FUGW/Vero cell line. (c) Fluorescence images of Vero/TMPRSS2 and Vero/MSPL cell lines after consecutively incubating 15 passages were acquired by the fluorescence microscope, using FUGW/Vero cells as control. (d) Fluorescence intensity of Vero/TMPRSS2. Vero/MSPL and Vero cells were detected by flow cytometry after 15 serial passages.
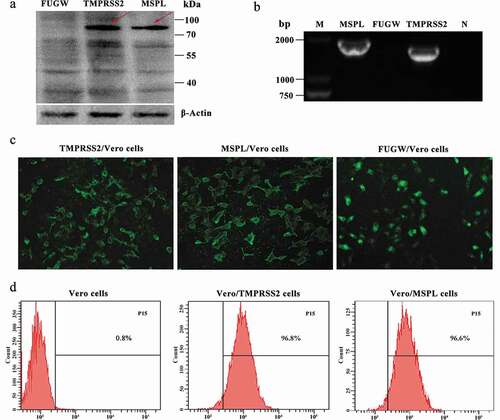
Figure 4. The propagation of cell-adapted PEDV strain LJB/03 P23 in Vero cells (with or without trypsin), Vero/TMPRSS2 and Vero/MSPL cells. Ultrathin sections of PEDV LJB/03-infected Vero cells at 24 h post-infection were prepared, and massive virus particles as shown by the arrow (Bar = 200 nm) were observed by the electron microscopy (a); PEDV LJB/03 particles in culture media as shown by the arrow (Bar = 200 nm) were observed by the transmission electron microscopy (b). (c) Trypsin-dependence of LJB/03 P23 was determined by RT-PCR assay with SYBR Premix EX Taq II (* p < 0.05, ** p < 0.01 compared to the Vero cells without trypsin group). (d) Replication kinetics of PEDV LJB/03 P23 in Vero cells (with or without trypsin), Vero/TMPRSS2, and Vero/MSPL cells were determined by RT-PCR, respectively. Vero cells (with 3 µg/mL or without trypsin), Vero/TMPRSS2, and Vero/MSPL cells were incubated with PEDV LJB/03 P23 at MOI = 0.01 and samples were harvested at 0, 12, 24, 36, 48, 60, 72, and 84 h post-infection, and then Viral RNA copy numbers were determined by RT-PCR. (e) PEDV LJB/03 P23 titers in Vero cells (with or without trypsin), Vero/TMPRSS2, and Vero/MSPL cells were determined by the plaque assay. Cells were incubated with PEDV LJB/03 P23 at MOI = 0.1 supplemented with 3 µg/mL trypsin (Vero cells) or PBS (Vero cells, Vero/TMPRSS2 and Vero/MSPL cells) and the viral titers (PFU/mL) were calculated by counting the syncytias. #P < 0.05, ##P < 0.01 as compared to Vero/TMPRSS2 cells.
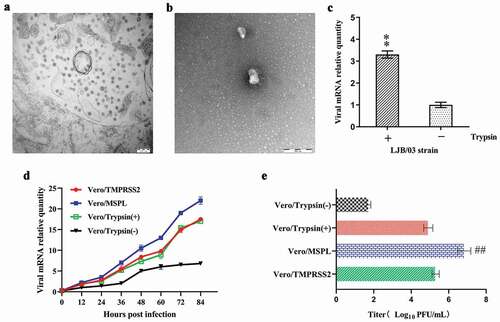
Figure 5. Proteolytic activation of PEDV S protein in Vero, Vero/TMPRSS2 and Vero/MSPL cells. (a) The expression of PEDV S genes in the Vero cells. Vero cells were transfected with PEDV-S plasmids (pCMV-HA-S encoding PEDV LJB/03 S protein with a HA tag) and the expression of PEDV S genes in the Vero cells were detected by western blot with anti-HA monoclonal antibody after 72 h post-transfection. Empty pCMV-HA plasmid as control. (b and c) Cleavage of PEDV S protein in Vero cells (with or with trypsin), Vero/TMPRSS2 and Vero/MSPL cells. Arrows indicate either uncleaved S protein (black arrows) or N-terminal cleavage S protein products (white arrow). Empty pCMV-HA plasmid as control.
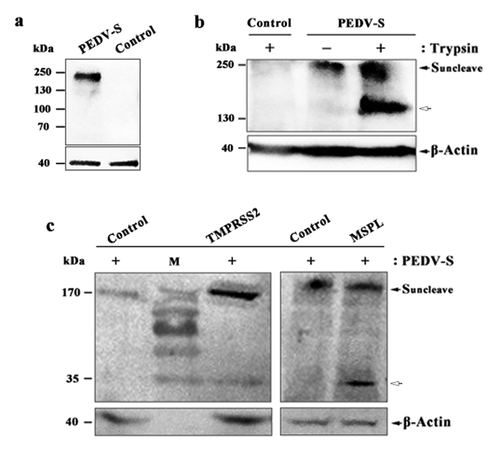
Figure 6. Trypsin-dependence and propagation of PEDV isolates 2013-A and NJ in Vero/TMPRSS2, Vero/MSPL cells and Vero cells (with or without trypsin). (a and b) Trypsin-dependence of 2013-A and NJ were determined by RT-PCR assay with the 2−∆∆Ct method as described above. Error bars indicate the standard error of three independent experiments and the viral mRNA relative quantity of the Trypsin(-) group set to 1. (* p < 0.05, ** p < 0.01,*** p < 0.001 compared to the Vero cells without trypsin). (c and d) The propagation of PEDV isolates 2013-A and NJ in Vero/TMPRSS2, Vero/MSPL, cells and Vero cells (with 3 µg/mL or without trypsin) was analyzed by RT-PCR at 72 h post-infection. The viral mRNA relative quantity of the Vero/Trypsin(-) set to 1 and Error bars indicate the standard error of the mean. (*P < 0.05, **P < 0.01 *** p < 0.001 compared to the Vero cells with trypsin; #P < 0.05, ##P < 0.01 as compared to Vero/TMPRSS2 cells).
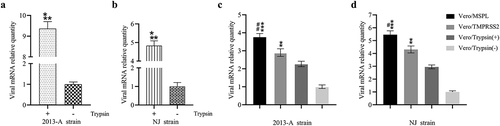
Figure 7. The cytopathic effects (CPEs) of Vero/TMPRSS2, Vero/MSPL, and Vero cells (without or with 3 μg/mL trypsin) incubated with PEDV isolates 2013-A and NJ. The Vero/TMPRSS2, Vero/MSPL, and Vero cells (without or with 3 μg/mL trypsin) were, respectively, incubated with PEDV isolates (2013-A and NJ) at an MOI = 0.01, followed by observation of CPEs. (a) CPEs observation of trypsin-dependent PEDV 2013-A at 24, 48, and 72 h post-infection; (b) CPEs observation of trypsin-dependent PEDV NJ at 24, 48, and 72 h post-infection. Arrows indicate syncytium.
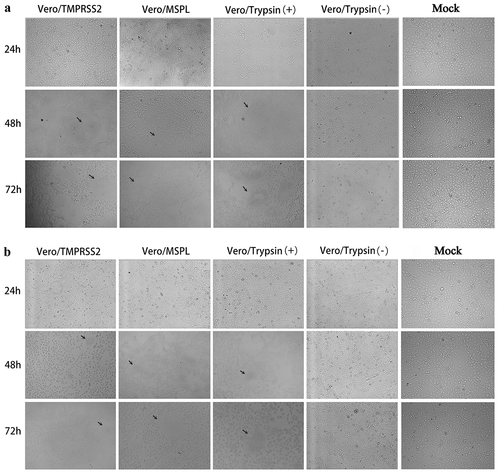
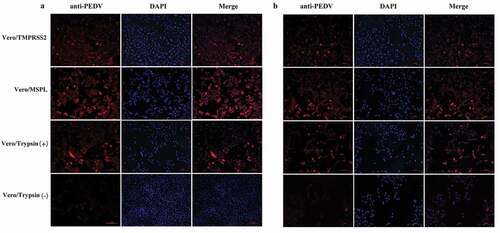
Figure 8. Immunofluorescence of Vero/TMPRSS2, Vero/MSPL, and Vero cells (without or with 3 μg/mL trypsin) infected by PEDV isolates 2013-A and NJ at an MOI = 0.01. (a) The fluorescence intensity was observed in trypsin-dependent PEDV 2013-A at 48 h post-infection. (b) The fluorescence intensity of trypsin-dependent PEDV NJ at 48 h post-infection.