Figures & data
Figure 1. Modulation of the autophagy pathway by coronaviruses and proposal of novel smart drug-loaded nanoparticles to target this pathway to combat COVID-19. Schematic shows how coronaviruses interact with autophagy. The NSP6 protein of SARS and MHV induces the formation of autophagosomes but confines their expansion and blocks their maturation into autolysosomes. A similar effect is observed by PLpro-TM of SARS. Human CoVs (HCoVs) via their NSPs, and MHV induce the formation of LC3-I-coated DMVs needed for viral RNA transcription and replication. MERS decreases the level of BECN1 (beclin 1) and blocks fusion of autophagosomes with lysosomes. Chloroquine/hydroxychloroquine, emtricitabine/tenofovir, interferon alfa-2b, lopinavir/ritonavir and ruxolitinib, which are all under clinical trial for treatment of SARS-CoV-2, induce autophagosome accumulation by blocking their maturation into autolysosomes. Thus, designing nanoparticles for the targeted delivery of these drug to avoid their off-target effects will provide safe and effective powerful tools to combat COVID-19. ATG14: autophagy related 14; DMV: double-membrane vesicles; EDEMosome: LC3-I-positive endoplasmic reticulum-derived vesicles exporting short-lived ERAD regulators; ER: endoplasmic reticulum; LC3-I: processed MAP1LC3; LC3-II: lipidated MAP1LC3; MERS: Middle East respiratory syndrome; MHV: murine gammaherpes virus; NSP6: non-structural protein 6; PIK3C3/VPS34: phosphatidylinositol 3-kinase catalytic subunit type 3; PIK3R4/VPS15: phosphoinositide-3-kinase regulatory subunit 4; PtdIns3 K: class III phosphatidylinositol 3-kinase; PLpro-TM: membrane-anchored papain-like protease; SARS: severe acute respiratory syndrome; ULK1 complex: unc-51 like autophagy activating kinase 1.
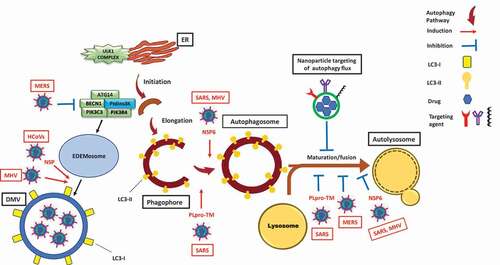
Table 1. Drugs under clinical trials against SARS-CoV-2 infection based on the World Health Organization report [Citation19], their autophagy-related mechanism of action, and their severe side-effects.
