Figures & data
Table 1. Bacterial strains and plasmids used in this study.
Table 2. Primers used in this study.
Figure 1. Screening of phagocytosis resistance transposon mutants. (a) Semiquantitative phagocytosis assay was performed for the initial screening of mutants with decreased anti-phagocytosis ability. Part of the results were presented. (b) A total of 3.0 × 105 Raw264.7 cells were infected individually with the transposon mutant strains or ZY05719 at an MOI of 10:1 for 1 h. CFUs of phagocytosed mutant strains and ZY05719 recovered from macrophages were determined (c) Confirmation of transposon insertion site. The transposon inserted gene had a 1300 bp size shift compared with the normal gene, which corresponds to the transposon sequence length. Lane M indicated the 5000 bp DNA marker. (d) Three SS2 ZY05719 transposon mutants with decreased anti-phagocytosis ability (M319, M431 and M71) were selected randomly and examined for their survival rate in whole swine blood. The survival ability of the 3 mutants decreased significantly compared to the wild-type strain. (e) Four SS2 ZY05719 transposon mutants with decreased anti-phagocytosis ability (M431, M604, M71 and M743) were selected randomly, and the survival ability in Raw264.7 cells were evaluated. The mutants showed decreased survival in Raw264.7 cells compared to the wild-type strain. Data represent the means with standard error of the mean of three samples. The statistical significance was determined by Student’s t test. * P < 0.05; ** P < 0.01; *** P < 0.001. (f) Virulence to Balb/c mice were determined. Three mutants M431, M319, M137 and wild-type ZY05719 were injected via intraperitoneal at a dose of 3 × 108 per mouse. Each group contained 8 mice. The mutants showed attenuated virulence to different extents compared with ZY05719.
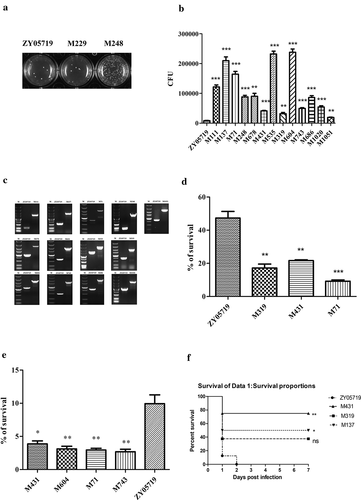
Table 3. Identification of transposon inserted genes of mutants with decreased anti-phagocytosis ability.
Figure 2. Phagocytosis, adhesion rate and virulence of knockout strains ΔMetQ and ΔWalR. (a) A total of 3.0 × 105 Raw264.7 cells were infected separately with the ΔMetQ, CΔMetQ or ZY05719 at an MOI of 10:1 for 1 h. CFUs of phagocytosed bacteria recovered from macrophages were determined. ΔMetQ showed M319 similar impairment in phagocytosis resistance. CΔMetQ restored the anti-phagocytosis ability to some extent. (b) A total of 3.0 × 105 Raw264.7 cells were infected separately with the ΔWalR, CΔWalR or ZY05719 at an MOI of 10:1 for 1 h. CFUs of phagocytosed bacteria recovered from macrophages were examined. ΔWalR were more susceptible to phagocytosis, whereas CΔWalR recovered the phenotype to the level of ZY05719. (c) Raw264.7 cells were pretreated with 1 μg/mL cytochalasin D for 30 min at 37°C under 5% CO2. Cells were infected with bacteria (ΔMetQ, ΔWalR, CΔMetQ, CΔWalR or wild-type ZY05719) at an MOI of 10:1 for 2 h. Adhesion to macrophages were examined. Except for ΔWalR, adhesion rates were not significantly different from that of ZY05719. Data are shown as the means and standard error of the mean from three independent experiments. * P < 0.05; ** P < 0.01; *** P < 0.001. (d) Balb/c mice were challenged with ΔMetQ, ΔWalR, CΔMetQ, CΔWalR or wild-type ZY05719 via intraperitoneal injection at a dose of 3 × 108 per mouse. Each group contained 8 mice. Virulence of ΔMetQ and ΔWalR were evaluated and the virulence to Balb/c mice were attenuated to different extent compared with ZY05719. The results were similar with the virulence of the corresponding transposon mutant strains M319 and M431. Complemented strains recovered the phenotype to the level of wild-type ZY05719. P < 0.05; ** P < 0.01; *** P < 0.001.
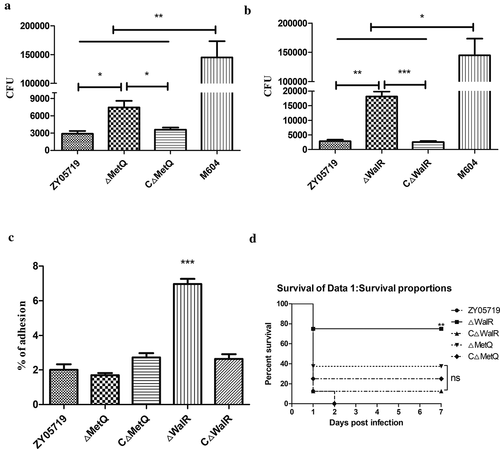
Figure 3. Growth kinetics in THY and CPS biosynthesis. (a) A subset of the 13 mutant strains were examined for their growth characteristics in THY at 37°C with vigorous shaking. There were no obvious differences in growth characteristics among mutants and wild-type ZY05719. Data are shown as the means with standard deviations from three independent experiments. (b) TEM images show the capsule of M431, M604 and ZY05719. Black arrows indicate the CPS of SS2, and the scale bars are 200 nm. CPS thickness of M431 and ZY05719 did not change obviously. CPS of M604 could hardly be visualized. The capsule thicknesses were quantified and shown in histogram. Data are shown as the means and standard error of the mean. * P < 0.05; ** P < 0.01; *** P < 0.001.
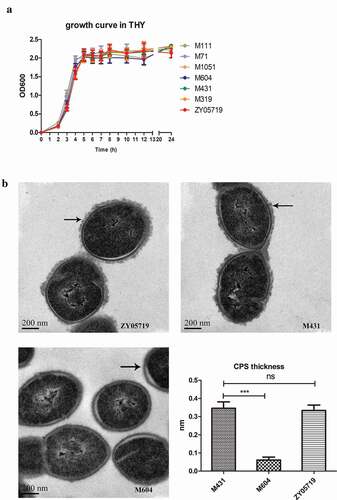
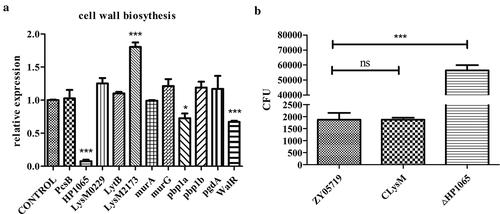
Figure 4. Peptidoglycan biosynthesis-related genes in M431. (a) Ten peptidoglycan biosynthesis-related genes were examined by qPCR for their mRNA levels in M431 and wild-type strain ZY05719. Levels of HP1065 and pbp1a decreased significantly in M431 compared with those in ZY05719, while levels of LysM2173 increased significantly. Each sample was assayed in two replicates and the data represented the means and standard error of the mean from three independent experiments. * P < 0.05; ** P < 0.01; *** P < 0.001. (b) Deletion of HP1065 (ΔHP1065) and overexpression of LysM2173 in wild-type strain ZY05719 (CLysM), were constructed and the phagocytosis assay was conducted. Raw264.7 cells were infected separately with the ΔHP1065, CLysM or ZY05719 at an MOI of 10:1 for 1 h. CFUs of phagocytosed bacteria recovered from macrophages were examined. Phagocytosed ΔHP1065 increased significantly compared with ZY05719. Whereas, engulfed CLysM did not change significantly compared with wild-type ZY05719. Data represent the means and standard error of the mean from three independent experiments. * P < 0.05; ** P < 0.01; *** P < 0.001.