Figures & data
Figure 1. Expression of QS-regulated P. aeruginosa virulence factors in vitro is growth phase dependent. (a) The CFU of P. aeruginosa strain PAO1 at different OD 600 nm values corresponding to different phases of growth in LB broth. (b) Exoprotease activities of PAO1 cultured in LB broth at different OD 600 nm values. (c–d) Transcription of QS-dependent lasB and rhlA genes in PAO1 and ∆lasI∆rhlI cultured in LB broth. Experiments were performed independently three times in triplicate. Mean ± standard error (SE) from a typical experiment are presented. *p < 0.05 (b–d) when compared against the lowest OD 600 nm by using the one-way ANOVA analysis. *p < 0.05 (b) when compared exoprotease activity in each sample against the sample with the lowest OD 600 nm by using the Tukey’s test. *p < 0.05 (c–d) when compared gene expression in PAO1 against ∆lasI∆rhlI at each OD 600 nm value by using the Tukey’s test. NC: LB alone.
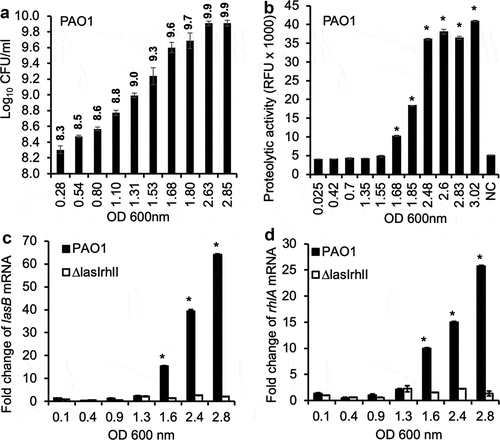
Figure 2. Exponential phase P. aeruginosa bacteria infect mouse lungs as successfully as their stationary phase counterparts. Anesthetized CD1 mice were intranasally inoculated with 2.5 × 10^7 CFU of early exponential phase (OD 0.63 at 600 nm), mid exponential phase (OD 1.13), and stationary phase (OD 2.73) P. aeruginosa PAO1. Mice were sacrificed between 0 to 24 hours post-infection for determination of bacterial burden and virulence gene expression. (a) P. aeruginosa burden in lung homogenates was enumerated 18 hours post-infection. Data are the mean CFU ± SE, n = 10 per group. (b) PAO1 burden in mouse lungs during the course of infection. Data are the mean CFU ± SE, n = 5 per group. (c–d) Changes in the transcription of virulence genes lasB (c) and rhlA (d) in PAO1 bacteria within the homogenates of infected mouse lungs at indicated time points. n = 5 per group. *p < 0.05 when compared the expression of lasB and rhlI at 6, 12 and 18-hour post-infection against Time 0 hour by using the one-way ANOVA analysis.
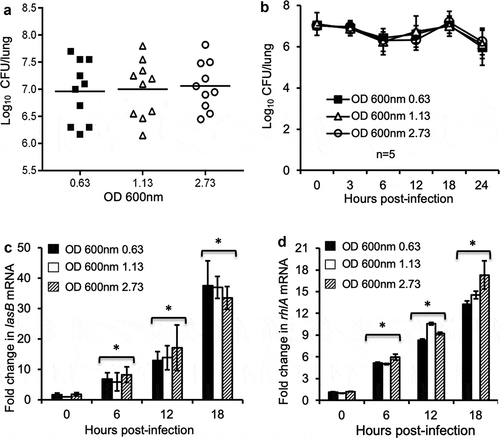
Figure 3. QS-regulated P. aeruginosa virulence factors are expressed prematurely in mouse BALF. P. aeruginosa strain PAO1 were grown to early exponential phase (OD 600 nm 0.63), mid exponential phase (OD 600 nm 1.13), and stationary phase (OD 600 nm 2.73). The cultured bacteria were washed 3 times at ice-cold sterile 0.9% NaCl with a 4°C microcentrifuge. Then, 2.5 × 107 (log10 7.4) CFU of washed PAO1 were cultured in BALF from healthy CD-1 mice at 37°C while shaking at 120 rpm and collected at different cell density from log10 8.1 to 9.1 CFU. For LB control, 2.5 × 107 washed stationary phase (OD 600 nm 2.73) PAO1 were cultured in LB and collected at cell density from log10 8.1 to 9.1. For 0.9% NaCl control, washed stationary phase (OD 600 nm 2.73) PAO1 diluted to cell density from log10 8.1 to 9.1 were incubated for 3 h respectively and then collected for detection. (a) Exoprotease activities of PAO1 in cultured supernatants. (b) Concentration of rhamnolipids released by PAO1 in cultured supernatants. (c–d) mRNA of lasB and rhlA genes were quantified by qPCR from PAO1 bacteria under indicated culture conditions. Experiments were performed independently three times in triplicate. (a-d) Mean ± SE from a typical experiment are presented. *p < 0.05 when compared BALF samples against both 0.9% NaCl and LB samples by using the one-way ANOVA analysis, as well as when compared each sample against 0.9% NaCl and LB by using the Tukey’s test. (e) Elastase B secreted by PAO1 into supernatant was analyzed by western blot visualized with anti-LasB primary antibody. Western blotting was performed twice with similar results. Expression level was normalized against bacterial numbers and quantified by densitometry and presented in the Fig. S1.
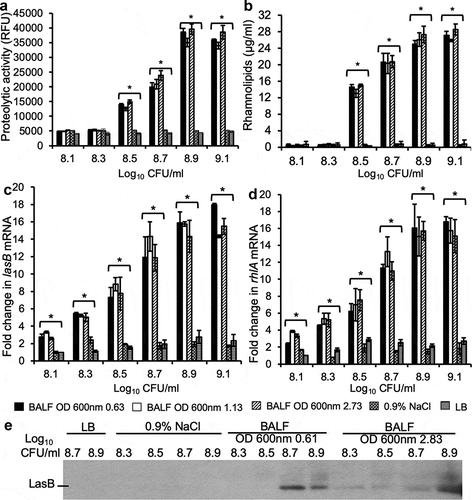
Figure 4. Mouse BALF promotes early expression of QS regulatory genes in P. aeruginosa. PAO1 bacteria were grown to early log (OD 600 nm 0.63), late log (OD 600 nm 1.13), and stationary phase (OD 600 nm 2.73), washed, and subcultured in 0.9% NaCl, LB and mouse BALF as described in . Transcription of various P. aeruginosa QS regulatory genes lasR (a), lasI (b), rhlR (c), and rhlI (d) were examined by qPCR. Experiments were performed independently three times in triplicate. Mean ± SE from a typical experiment are presented. *p < 0.05 when compared BALF samples against the 0.9% NaCl and LB samples by using the one-way ANOVA analysis, as well as when compared each BALF sample against 0.9% NaCl and LB by using the Tukey’s test.
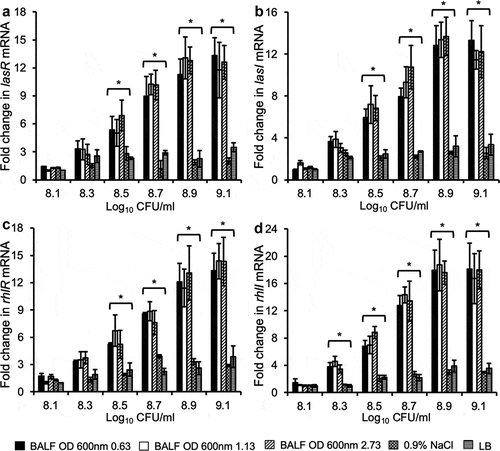
Figure 5. Remodeling of virulence expression in exponential phase P. aeruginosa by mouse BALF is dependent on LasR-LasI and RhlR-RhlI QS circuits. P. aeruginosa PAO1 bacteria were grown to early exponential phase (OD 600 nm 0.6), washed, diluted to indicated concentrations, and cultured in mouse BALF as described in . (a) Exoprotease activity of PAO1 and ∆lasI∆rhlI mutant were compared in mouse BALF. (b) The concentration of rhamnolipids released by PAO1 or ∆lasI∆rhlI growing in mouse BALF. Experiments were performed independently three times in triplicate. Mean ± SE from a typical experiment are presented. *p < 0.05 (a–b) when compared PC and PAO1 samples against ∆lasI∆rhlI by using the one-way ANOVA analysis, as well as when compared PC or PAO1 against individual ∆lasI∆rhlI samples by using the Tukey’s test. (c) LasB expressed by PAO1 or ∆lasI∆rhlI in mouse BALF was analyzed by western blot using anti-LasB antibody. PC was positive control from PAO1 grown in LB to late stationary phase (OD 600 nm 2.85). Western blotting was performed twice with similar results. LasB expression level was normalized against bacterial numbers and quantified by densitometry and presented in the Fig. S2.
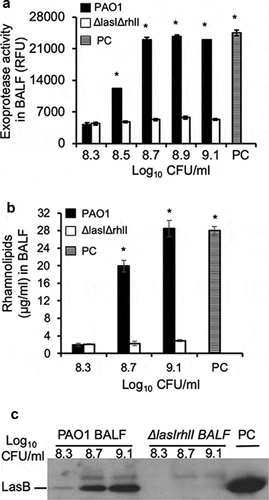
Figure 6. Pulmonary surfactant phospholipids are responsible for remodeling of QS-regulated virulence factor expression. P. aeruginosa PAO1 was grown to early exponential phase (OD 600 nm 0.6) and cultured in mouse BALF, BALF pre-extracted with phenol:chloroform:isoamyl alcohol (BALF+phenol) or pre-digested with PLA2 (BALF+PLA2), or BALF with exogenously supplied DPPC (100 µg/ml) after phenol:chloroform:isoamyl alcohol extraction (BALF+phenol+DPPC) or PLA2 digestion followed by heat inactivation (BALF+PLA2+DPPC), as described in . Culture supernatants were collected at different cell density of log10 8.3, 8.7, and 9.1, respectively, for determination of proteolytic activities and rhamnolipid production. (a-b) Exoprotease activities. (c–d) rhamnolipid production. Experiments were performed independently three times in triplicate. Mean ± SE from a typical experiment are presented. *#p < 0.05 when compared proteolytic activities and rhamnolipid production against of BALF+phenol or BALF+PLA2 by using the one-way ANOVA analysis, as well as within individual data set by using the Tukey’s test.
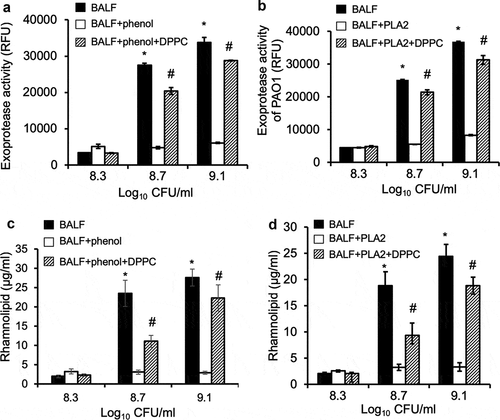
Figure 7. Dose-dependent restoration of proteolytic activities and rhamnolipid production by dipalmitoylphosphatidylcholine. P. aeruginosa PAO1 bacteria were grown to early exponential phase (OD 600 nm 0.6, log10 7.4 CFU ml−1), washed and cultured as described as Fig 3 to cell density of log10 CFU 9.1 in mouse BALF, BALF pre-extracted with phenol:chloroform:isoamyl alcohol alone, or supplemented with indicated concentrations of DPPC after phenol:chloroform:isoamyl alcohol extraction. (a) Proteolytic activities. (b) rhamnolipid production. *p < 0.05 when compared exoprotease activity and rhamnolipid production against phenol:chloroform:isoamyl alcohol-extracted BALF alone by using the one-way ANOVA analysis, as well as by comparing each sample against phenol:chloroform:isoamyl alcohol-extracted BALF alone by using the Tukey’s test.
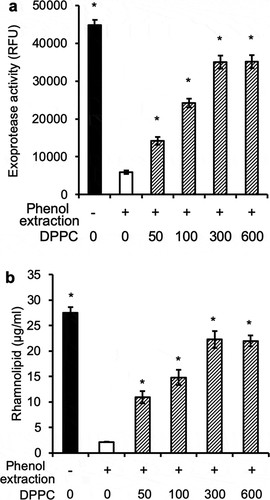
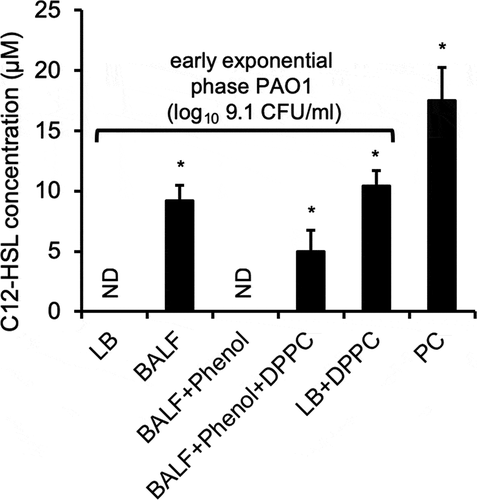
Figure 8. DPPC alone induces the premature production of homoserine lactone C12-HSL by P. aeruginosa. The levels of C12-HSL as determined by GC-MS produced in supernatant of exponential phase PAO1 (OD 600 nm 0.6, log10 9.1 CFU/ml) grown in LB, BALF, phenol:chloroform:isoamyl alcohol-extracted BALF (BALF+phenol), phenol:chloroform:isoamyl alcohol-extracted BALF supplemented with 100 µg/ml DPPC (BALF+phenol+DPPC), and LB + 100 µg/ml DPPC, as described in Fig 7. PC: Supernatants of stationary phase PAO1 grown in LB (OD 600 nm 2.85, log10 9.9 CFU/ml) was used as positive control. ND: Not detectable. Multiple attempts to detect C4-HSL with GC-MS analysis were unsuccessful (data not shown). *p < 0.05 when compared C12-HSL levels against exponential phase PAO1 grown in LB alone and in BALF+phenol by using the one-way ANOVA analysis, as well as within each data set by using the Tukey’s test.