Figures & data
Figure 1. The TMD sequence of EspB is critical for the ability of the protein to be secreted by the T3SS. (a) Protein secretion profiles of EPEC strains grown under T3SS-inducing conditions: wild-type (WT) EPEC, ΔescN (a T3SS ATPase mutant), ΔespB, and ΔespB expressing EspBwt-His. The secreted fractions were concentrated from the supernatants of bacterial cultures and analyzed by SDS-PAGE and Coomassie blue staining. The T3SS-secreted translocators EspA, EspB, and EspD are marked on the right of the gel. Also indicated is the location of EspC, which is not secreted via the T3SS. (b) Bacterial pellets and supernatants were analyzed by SDS-PAGE and western blot with an anti-His antibody to confirm EspBwt-His expression and secretion. (c) Schemes of the EspBwt and EspB7L9A proteins with their corresponding TMD sequences and the calculated apparent free energy differences(ΔGapp). Protein secretion profiles and bacterial pellets of EPEC WT, ΔescN, and ΔespB strains only or expressing either EspBwt-His or EspB7L9A-His, grown under T3SS-inducing conditions were analyzed similar to panel A
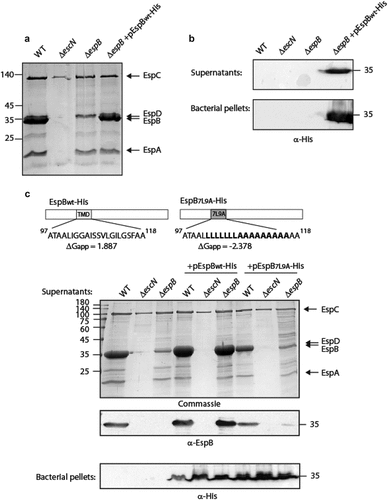
Figure 2. The localization of EspB and its interaction with CesAB. (a) ΔespB EPEC expressing either EspBwt-His or EspB7L9A-His were grown under T3S-inducing conditions, supernatants were collected, and whole-cell extracts were fractionated into periplasmic (p), cytoplasmic (c), and membranal (m) fractions. The samples (30 µg) were analyzed by SDS-PAGE and western blotting using an anti-His antibody. (b) To confirm correct bacterial fractionation, the western blots were probed with anti-DnaK (cytoplasmic marker), anti-MBP (periplasmic marker), and anti-intimin (membranal marker) antibodies. (c) Elution fractions of EPEC ΔespB expressing EspBwt alone, CesAB-His alone or CesAB-His in combination with EspBwt or EspB7L9A, were loaded on SDS-PAGE and analyzed by western blotting with anti-His and anti-EspB antibodies
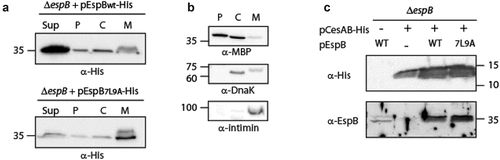
Figure 3. The TMD sequences of EspD are crucial for the secretion of the protein by the T3SS. (a) Protein secretion profiles of EPEC strains grown under T3SS-inducing conditions: WT EPEC, ΔescN, ΔespD, and ΔespD expressing either EspDwt-35His, EspDTMD1-35His, and EspDTMD2-35His. The secreted fractions were concentrated from the supernatants of bacterial cultures and analyzed by SDS-PAGE and Coomassie blue staining. The T3SS-secreted translocators EspA, EspB, and EspD are marked on the right of the gel. Also indicated is the location of EspC, which is not secreted via the T3SS. The calculated ΔGapp of each TMD sequence is presented. (b) Bacterial pellets and supernatants were analyzed by SDS-PAGE and western blot with an anti-His antibody to confirm EspD expression and secretion. (c) ΔespD EPEC expressing either EspDwt-35His (labeled WT), EspDTMD1-35His (labeled TMD1), or EspDTMD2-35His (labeled TMD2) were grown under T3S-inducing conditions, supernatants were collected, and whole-cell extracts were fractionated into periplasmic (p), cytoplasmic (C), and membranal (m) fractions. The samples (30 µg) were analyzed by SDS-PAGE and western blot using an anti-His antibody. To confirm correct bacterial fractionation, the western blots were probed with anti-DnaK (cytoplasmic marker), anti-MBP (periplasmic marker), and anti-intimin (membranal marker) antibodies
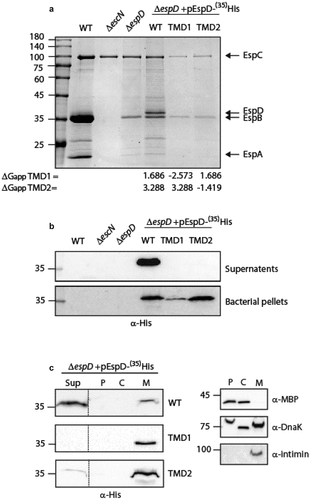
Figure 4. Replacement of EspB TMD by TMD sequences of Tir preserves the ability of EspB to be secreted by the T3SS. (a) Protein secretion profiles of EPEC WT, ΔescN, and ΔespB strains, and the same strains expressing either EspBwt-His, EspBTir1-His, or EspBTir2-His grown under T3SS-inducing conditions. The secreted fractions were concentrated and analyzed by SDS-PAGE and Coomassie blue staining. The location of the T3SS translocators EspA, EspB, EspD, and EspC (which is not secreted via the T3SS), are marked on the right of the gel. The calculated ΔGapp of each TMD sequence is presented. (b) Bacterial pellets and supernatants were analyzed by SDS-PAGE and western blot with an anti-His antibody. EspBTir1-His and EspBTir2-His were detected in the supernatants of WT and ΔespB strains, but not when expressed in the ΔescN mutant
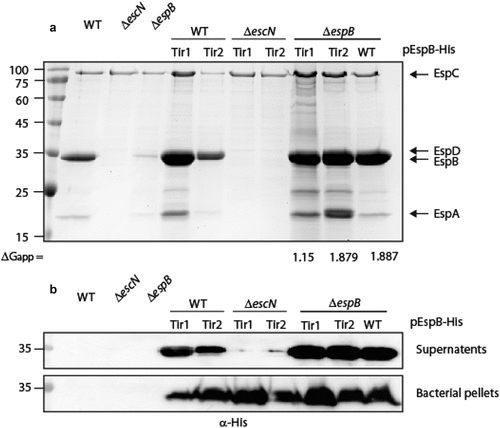
Figure 5. The TMD sequence of EspB is crucial for the activity of the protein. (a) Bacterial pellets and supernatants of EPEC WT, ΔescN, ΔespB, and ΔespB that express unlabeled EspBwt, EspB7L9A EspBTir1, or EspBTir2 were grown under T3SS-inducing conditions. The samples were analyzed by SDS-PAGE and western blot with an anti-EspB antibody. (b) Proteins extracted from HeLa cells infected with WT, ΔescN, ΔespB, and ΔespB expressing EspBwt, EspB7L9A EspBTir1 or EspBTir2. The samples were subjected to western blot analysis using anti-JNK and anti-actin (loading control) antibodies. JNK and its degradation fragments are indicated at the right of the gel. WT EPEC showed massive degradation of JNK relative to the uninfected sample and the samples infected with ΔescN or ΔespB mutant strains. The EPEC ΔespB strain complemented with EspBwt showed a similar JNK degradation profile as WT EPEC, while the ΔespB strain complemented with EspB7L9A EspBTir1 or EspBTir2 showed JNK degradation profiles similar to those of ΔescN and ΔespB strains
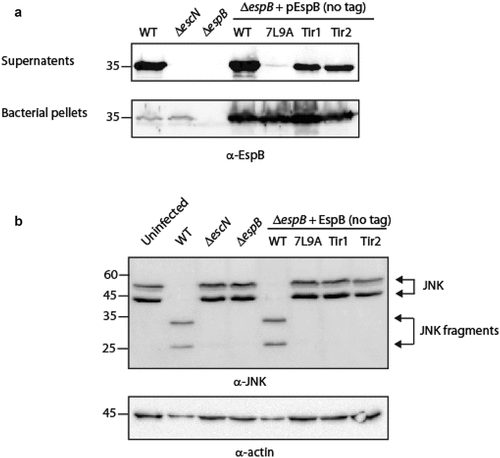
Figure 6. EspB’s TMD is critical for the ability of EspB, EspD and Tir to translocate into host cell membranes. HeLa cells were infected with EPEC WT, ΔescN, ΔespB, and ΔespB that express EspBwt, EspB7L9A, EspBTir1, or EspBTir2, washed, and lysed. Cellular samples were subjected to SDS-PAGE and western blot analysis using anti-EspB, anti-EspD, anti-Tir, and anti-actin (loading control) antibodies. EspB, EspD and Tir translocation was detected in HeLa cells infected with EPEC WT or ΔespB expressing EspBwt, but not in HeLa cells infected with ΔespB expressing either EspB7L9A, EspBTir1 or EspBTir2.
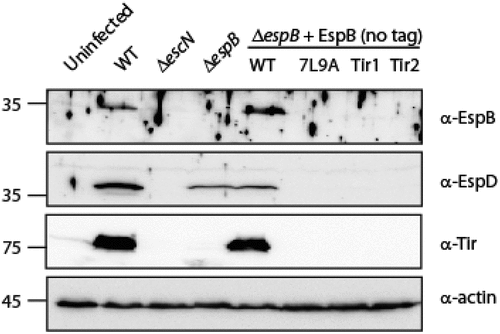
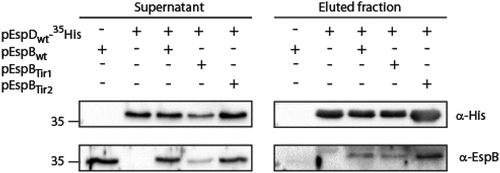
Figure 7. EspB TMD is not involved in EspB-EspD interaction. Supernatants of EPEC ΔespB expressing EspBwt alone, EspDwt-35His alone or in combination with EspBwt, EspBTir1 or EspBTir2 were subjected to co-purification using Ni-beads. Samples of supernatants and elution fractions were loaded on SDS-PAGE and analyzed by western blotting with anti-His and anti-EspB antibodies. Supernatants confirmed proper protein secretion to the extracellular medium. EspBwt, EspBTir1 or EspBTir2 co-eluted with EspDwt-35His