Figures & data
Table 1. Bacterial strains derived from the USA300 clone and used in this study. All bacterial strains were provided by the French national reference center for staphylococci [Citation27]
Table 2. Sequences of primers used for RT-qPCR
Figure 1. Cytotoxicity of PSMs on primary human keratinocytes. Keratinocytes were exposed to synthetic and purified PSM Fα3 (N-formylated) or purified PSM α3 (a-b) and to supernatants from a total PSM-producing strain (Total PSMs), a deleted PSMα1-4 strain (Δ PSMα1-4) or a deleted total PSM strain (Δ Total PSMs) of S. aureus (c-d) for 24 h. Cell viability was evaluated using either a XTT assay (a; c) or measuring the lactate dehydrogenase released (b; d). Data are represented as mean + standard error of mean (SEM) of at least three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
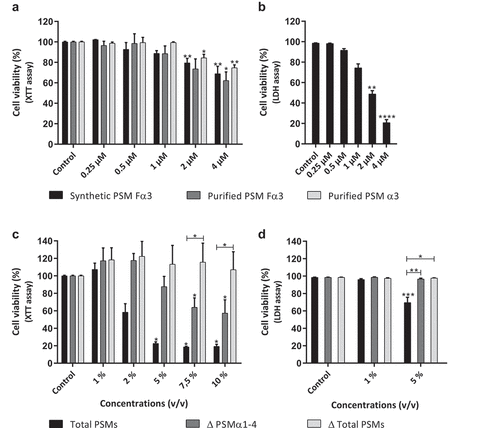
Figure 2. Synthetic and purified PSM α3 from S. aureus triggered pro-inflammatory chemokine expression in keratinocytes at 3 h post-stimulation. mRNA fold increase of CXCL1 (a), CXCL2 (b), CXCL3 (c), CXCL5 (d), CXCL8 (e) and CCL20 (f) was quantified in keratinocytes at 3 h post-stimulation with synthetic and purified PSM Fα3 or purified PSM α3 as compared to unstimulated keratinocytes. Data are represented as mean + SEM of at least three independent experiments. *p < 0.05, **p < 0.01
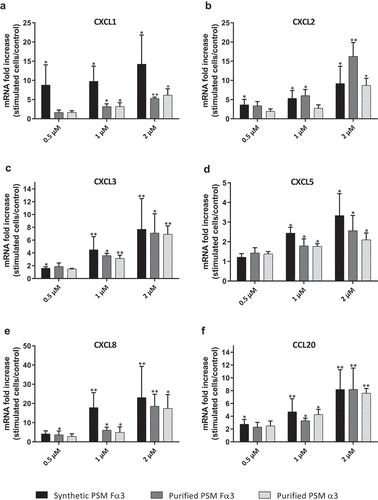
Figure 3. Synthetic and purified PSM α3 from S. aureus stimulated pro-inflammatory cytokine expression in keratinocytes at 3 h post-stimulation. mRNA fold increase of IL-6 (a), TNF-α (b), IL-1α (c), IL-1β (d) and IL-36γ (e) were quantified in keratinocytes at 3 h post-stimulation with synthetic and purified PSM Fα3 or purified PSM α3 as compared to unstimulated keratinocytes. Data are represented as mean + SEM of at least three independent experiments. *p < 0.05, **p < 0.01
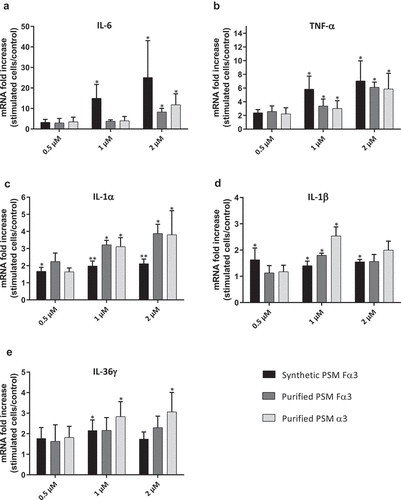
Figure 4. PSMs from S. aureus induced cytokine and chemokine production in keratinocytes at 24 h post-stimulation. Keratinocytes were exposed to synthetic and purified PSM Fα3 and purified PSM α3 for 24 h. CXCL8 (a), CCL20, TNF-α and IL-6 (b) concentrations was assessed in culture supernatants. Data are represented as mean + SEM of at least three independent experiments. *p < 0.05, **p < 0.01
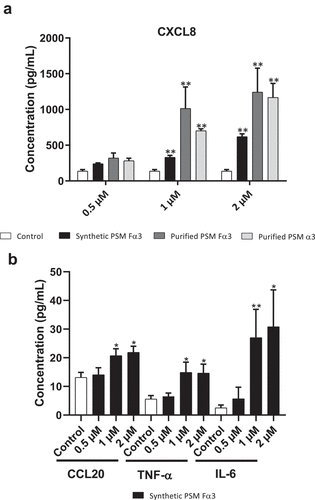
Figure 5. Secretion of PSMs α by S. aureus was crucial to promotion of pro-inflammatory chemokine and cytokine expression in keratinocytes at 3 h post-stimulation. mRNA fold increase of CXCL1, CXCL2, CXCL3, CXCL5, CXCL8, CCL20, IL-6, TNF-α, IL-1α, IL-1β and IL-36γ was quantified in keratinocytes at 3 h post-stimulation with 1% (v/v) supernatants from total PSM-producing strain (Total PSMs), PSMα1-4-deficient strain (Δ PSMα1-4) or total PSMs-deficient strain (Δ Total PSMs) of S. aureus as compared to unstimulated keratinocytes. Data are represented as mean + SEM of at least three independent experiments. *p < 0.05, **p < 0.01
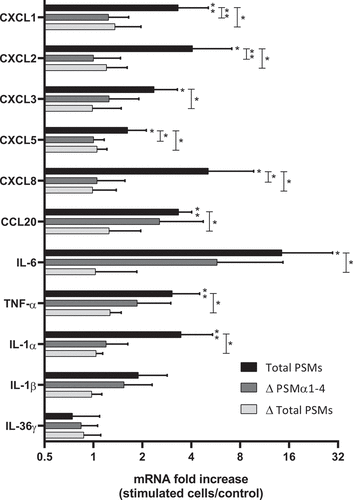
Figure 6. Histopathology of 3D control and stimulated human skin explants. Skin sections of explants were stained with Hematein-Eosin-Safran (HES) at 10 min (a) and 24 h post-incubation without (b) or with supernatants containing all PSMs (c), supernatants from PSMα1-4-deficient strain (d) and supernatants from total PSM-deficient strain (e) deposited on a paper disk on the top of explants for 24 h. Scale bar = 20 µm
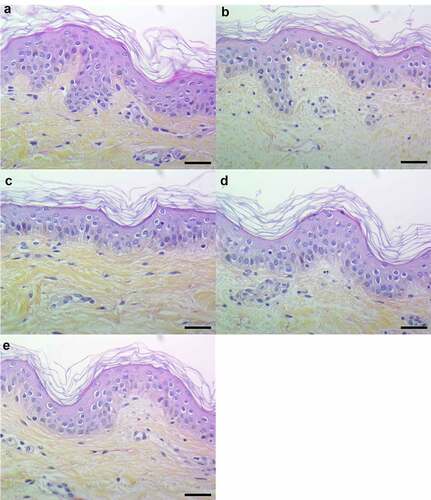
Figure 7. Topical and basal layer stimulations with PSMs from S. aureus of ex vivo human skin explants increased the expression of pro-inflammatory chemokines. Same volume (20 µL) of supernatants from total PSM-producing strain (Total PSMs), PSMα1-4-deficient strain (Δ PSMα1-4) or total PSM-deficient strain (Δ Total PSMs) of S. aureus were added directly to the culture medium (basal layer stimulation) or were deposited on a paper disk on the top of explants (topical stimulation) for 24 h. mRNA fold increase of CXCL1 (a), CXCL2 (b), CXCL3 (c), CXCL5 (d), CXCL8 (e) and CCL20 (f) was quantified as compared to untreated explants. Data are represented as mean + SEM of at least three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001
Figure 8. Topical and basal layer stimulations with PSMs from S. aureus of ex vivo human skin explants increased the expression of pro-inflammatory cytokines. Same volume (20 µL) of supernatants from total PSM-producing strain (Total PSMs), PSMα1-4-deficient strain (Δ PSMα1-4) or total PSM-deficient strain (Δ Total PSMs) of S. aureus were added directly to the culture medium (basal layer stimulation) or deposited on a paper disk on the top of explants (topical stimulation) for 24 h. mRNA fold increase of IL-6 (a), TNF-α (b), IL-1α (c), IL-1β (d) and IL-36γ (e) was quantified as compared to untreated explants. Data are represented as mean + SEM of at least three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001
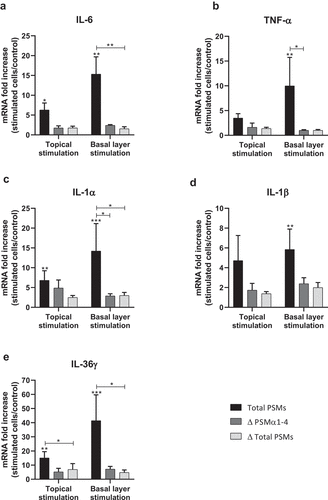
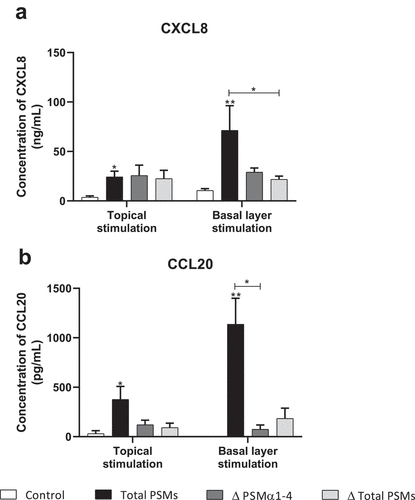
Figure 9. Topical and basal layer stimulations with PSMs from S. aureus of ex vivo human skin explants increased chemokine production. Supernatants from total PSM-producing strain (Total PSMs), PSMα1-4-deficient strain (Δ PSMα1-4) or total PSM-deficient strain (Δ Total PSMs) of S. aureus were added directly to the culture medium (basal layer stimulation) or deposited on a paper disk on the top of explants (topical stimulation) for 24 h. CXCL8 (a) and CCL20 (b) concentrations was evaluated by ELISA assays in culture supernatants. Data are represented as mean + SEM of at least three independent experiments. *p < 0.05, **p < 0.01