Figures & data
Figure 1. Regulatory network of the virulence factors in B. pertussis. BvgS is a sensor-kinase that is active by default at a temperature of 37°C. Its autophosphorylation is followed by the phosphorylation of its cognate response regulator BvgA. Phosphorylated BvgA triggers the expression of vir-activated genes (vags). BvgS responds to chemical stimuli such as sulfate or nicotinate ions by switching off the phosphorylation cascade, which increases the expression of vir-repressed genes (vrgs). BvgR is the product of a vag and hydrolyzes c-di-GMP to GMP. c-di-GMP affects the activity of the RisAK two-component system by binding to the response regulator RisA. The sensor-kinase RisS is truncated and nonfunctional in B. pertussis, and the partner of RisA is the sensor-kinase RisK. The targets of RisA depend on its phosphorylation and on the concentration of c-di-GMP. Non-phosphorylated RisA bound to c-di-GMP represses the expression of iron-related genes. With both c-di-GMP and phosphorylation RisA induces the expression of the vrgs and represses that of the vags and of the mobility genes, whereas in the absence of c-di-GMP, RisA induces the expression of other sets of genes depending on its phosphorylation. The sensor-kinase PlrS responds to CO2 by phosphorylating the response regulator PlrR. The regulon of PlrSR is unknown, but one of its target(s) interact(s) directly or indirectly with the BvgAS system in an uncharacterized manner. BrpL is a vag coding for a sigma factor that triggers the expression of the T3SS. BtrA antagonizes the activity of BrpL by titrating it in a BtrA/BrpL complex, which results in the repression of the T3SS and the mobility genes and the overexpression of certain vags. The dotted arrows represent interactions identified in B. bronchiseptica and suspected in B. pertussis.
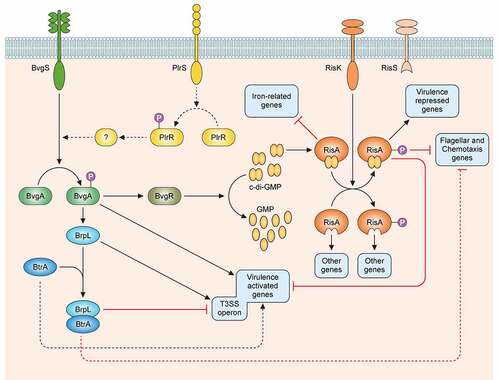
Table 1. Major virulence factors of B. pertussis.
Figure 2. Anti-immune strategies of B. pertussis. (a) Upon infection, several virulence factors (FHA, Prn, Tcf, Fim, BteA, ACT, PTX) regulated by the BvgA/S two-component system (Bvg+ bacteria) enable B. pertussis adherence to epithelial cells of the nasal mucosa. The role for ACT in adherence is likely indirect. Some bacteria may invade epithelial cells. (b) TCT is internalized by epithelial cells and, along with LOS, induces the release of NO by the intoxicated cells, which is a major destructor of epithelial cells. ACT and BteA may also contribute to the disruption of the epithelial barrier. (c) Once the epithelial barrier is broken and submucosal glands have fired, iron may be delivered onto the surface of epithelial cells by the plasma exudate [Citation234]. In response to the infection, the respiratory epithelium may produce highly sulfated mucins. Sulfate might be released from mucins by glycosyltransferases of B. pertussis and commensal bacteria [Citation235,Citation236], which might induce the Bvgi phase and hence trigger biofilm formation (d), thereby allowing long-term colonization. Up-regulation of the pendrin anion exchanger of epithelial cell drives the production of mucus, which along with ciliary damage, reduces mechanical clearance of the bacteria. (e) B. pertussis toxins, in particular PTX, suppress the early recruitment of immune cells. PTX prevents neutrophil chemotaxis indirectly by inhibiting the production of neutrophil-attracting chemokines KC, LIX and MIP-2 by alveolar macrophages (AM) and epithelial cells following disruption of TLR-4 signaling. (f) ACT may elicit reprogramming of infiltrating monocytes or alveolar macrophages to less bactericidal and short-lived monocyte-like cell types. Alternatively or additionally, FHA may enhance non-opsonic CR3-phagocytosis to escape effective clearance mediated by FcR recognition and suppresses – along with BrkA, LOS and ACT, and most of all Vag8 – complement-mediated killing (not represented). Expression of MgtC may enable the bacterium to escape opsonophagocytic killing by preventing phagosome-lysosome fusion, while ACT, PTX, FHA and Prn dampen the antimicrobial activity of the phagocytes, including oxidative stress and release of antimicrobial peptides (AMP). (g) To further avoid clearance by professional phagocytes, B. pertussis virulence factors such as ACT could trigger phagocyte apoptosis. (h) Several virulence factors may contribute to the accumulation of IFN-α-expressing DCs in the lungs that mediate early suppression of Th17 cell differentiation. (i) Intraepithelial DCs (and other myeloid cells) are reprogrammed to up-regulate IL-10 and down-regulate IL-12 production in order to favor the differentiation of regulatory T cells (Tr), Th2 and Th17 cells over Th1 cells. (j) ACT-intoxicated DCs are impaired in their capacity to stimulate T cells. The arrival of potentially tolerogenic DCs into the draining lymph nodes may alter cellular protective immune responses against B. pertussis. (k) Additionally, PTX is able to suppress serum antibody responses
![Figure 2. Anti-immune strategies of B. pertussis. (a) Upon infection, several virulence factors (FHA, Prn, Tcf, Fim, BteA, ACT, PTX) regulated by the BvgA/S two-component system (Bvg+ bacteria) enable B. pertussis adherence to epithelial cells of the nasal mucosa. The role for ACT in adherence is likely indirect. Some bacteria may invade epithelial cells. (b) TCT is internalized by epithelial cells and, along with LOS, induces the release of NO by the intoxicated cells, which is a major destructor of epithelial cells. ACT and BteA may also contribute to the disruption of the epithelial barrier. (c) Once the epithelial barrier is broken and submucosal glands have fired, iron may be delivered onto the surface of epithelial cells by the plasma exudate [Citation234]. In response to the infection, the respiratory epithelium may produce highly sulfated mucins. Sulfate might be released from mucins by glycosyltransferases of B. pertussis and commensal bacteria [Citation235,Citation236], which might induce the Bvgi phase and hence trigger biofilm formation (d), thereby allowing long-term colonization. Up-regulation of the pendrin anion exchanger of epithelial cell drives the production of mucus, which along with ciliary damage, reduces mechanical clearance of the bacteria. (e) B. pertussis toxins, in particular PTX, suppress the early recruitment of immune cells. PTX prevents neutrophil chemotaxis indirectly by inhibiting the production of neutrophil-attracting chemokines KC, LIX and MIP-2 by alveolar macrophages (AM) and epithelial cells following disruption of TLR-4 signaling. (f) ACT may elicit reprogramming of infiltrating monocytes or alveolar macrophages to less bactericidal and short-lived monocyte-like cell types. Alternatively or additionally, FHA may enhance non-opsonic CR3-phagocytosis to escape effective clearance mediated by FcR recognition and suppresses – along with BrkA, LOS and ACT, and most of all Vag8 – complement-mediated killing (not represented). Expression of MgtC may enable the bacterium to escape opsonophagocytic killing by preventing phagosome-lysosome fusion, while ACT, PTX, FHA and Prn dampen the antimicrobial activity of the phagocytes, including oxidative stress and release of antimicrobial peptides (AMP). (g) To further avoid clearance by professional phagocytes, B. pertussis virulence factors such as ACT could trigger phagocyte apoptosis. (h) Several virulence factors may contribute to the accumulation of IFN-α-expressing DCs in the lungs that mediate early suppression of Th17 cell differentiation. (i) Intraepithelial DCs (and other myeloid cells) are reprogrammed to up-regulate IL-10 and down-regulate IL-12 production in order to favor the differentiation of regulatory T cells (Tr), Th2 and Th17 cells over Th1 cells. (j) ACT-intoxicated DCs are impaired in their capacity to stimulate T cells. The arrival of potentially tolerogenic DCs into the draining lymph nodes may alter cellular protective immune responses against B. pertussis. (k) Additionally, PTX is able to suppress serum antibody responses](/cms/asset/067af83a-e470-4564-9ac8-5b4cb2d5e45c/kvir_a_1980987_f0002_oc.jpg)
Data Availability statement:
I do not have a DOI yet, and since this is an invited signature review, I don’t think this applies here
