Figures & data
Figure 1. Dendritic cell subsets. Dendritic cells (DCs) are subdivided into different subsets that display different phenotypes and functions. Importantly, hematopoietic stem cells (HSCs) are the main precursors of the different DCs lineages. On the one hand, HSCs generate lymphoid progenitors that produce the differentiation of steady-state DCs that are subdivided into plasmacytoid DCs (pDCs) and conventional DCs (cDCs). Plasmacytoid DCs (pDCs) are characterized by the expression of high levels of type-I interferons (IFN-I) and the presence of high amounts of rough endoplasmic reticulum (RER). cDCs display a high expression of CD11c and MHC-II molecules. Moreover, cDCs are subclassified into cDC1 and cDC2, with cDC1 expressing high levels of the C-type lectin domain-containing 9A (Clec9A) and the chemokine XC receptor 1 (XCR1), while cDC2 express on their cell surface high levels of CD1c and the signal regulatory protein α (SIRPα). On the other hand, HSCs are the precursors of myeloid progenitors, which can be differentiated into monocytes and later into inflammatory DCs (inf-DCs), also called monocyte-derived DCs (MoDCs). Inf-DCs characterized by the expression of high levels of MHC-II, CD11c and costimulatory molecules (CD40, CD80, and CD86). Finally, langerhans cells (LCs) are specifically located in the epidermis and originate from monocyte precursors and embryonic precursors. LCs present langerin (CD207) expression and the expression of birbeck granules in the cytoplasm
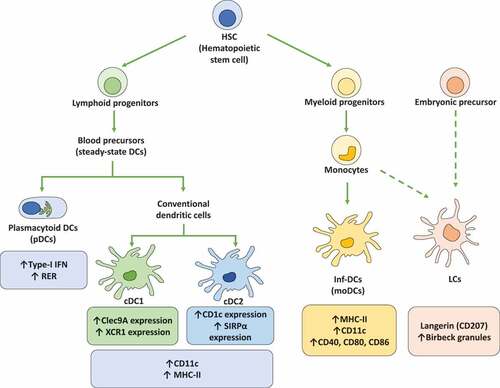
Table 1. Herpes simplex virus proteins interfere with DC functionality
Figure 2. Herpes simplex viruses modulate early antiviral responses in dendritic cells. The toll-like receptor (TLR) responses to HSV infections in DCs is indicated on the left. HSV infection produces TLR2 and TLR9 activation. TLR2 signaling is modulated by HSV-2 deoxyuridine triphosphate nucleotidohydrolases (dUTPases) proteins promoting NF-κB activation and expression of proinflammatory cytokines IL-6 and IL-8. Moreover, in the endosome, TLR9 can sense HSV-1 and HSV-2 dsDNA and elicit myeloid differentiation factor-88 (MyD88) signaling cascades promoting the secretion of high levels of IFN-α, as well as the expression of TNF-α and IL-6. On the other hand, HSV-1 can inhibit TBK-1 activation through increased synthesis of the tripartite motif-containing-30α (TRIM30α), a protein that negatively regulates the activation of NF-κB signaling. Moreover, the HSV-1 vhs and γ134.5 proteins block TLR-mediated NF-κB activation. Also, γ134.5 can abolish IFN-I production through direct binding to TANK-binding kinase-1 (TBK-1), which prevents the interferon regulatory factor-3 (IRF3) phosphorylation and their nuclear translocation. On the right, interferon responses to HSV infections. HSV-1 can inhibit the signal transducer and activator of transcription-1 (STAT1) protein associated with the type-I IFN receptor (IFNAR) and type-III IFN receptor (IFNLR) that prevent the translocation of STAT1 complexes into the nucleus and the activation of the transcription of interferon-stimulated genes (ISG)
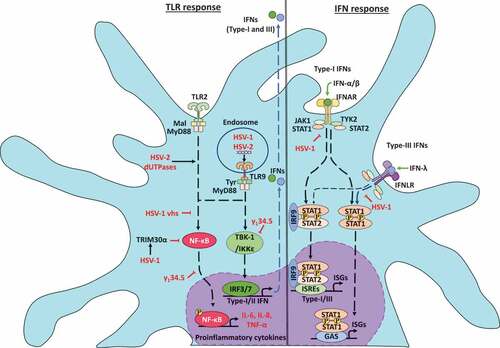
Figure 3. Dendritic cell cellular responses in response to infections with herpes simplex viruses. A. Apoptosis activation in dendritic cells infected by HSVs. A.1 Modulation of the extrinsic apoptosis pathway in DCs infected by HSVs. HSV-1 promotes the expression of death ligands, such as TNF-α and the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) recognized by the tumor necrosis factor receptor (TNFR), promoting a signaling cascade that concludes in the activation of caspase-8 and caspase-3 proteins. The activation of caspase-8 is negatively modulated by the cellular FLICE-inhibitory protein (c-FLIP). However, HSV-1 infection produces the inhibition of c-FLIP and the subsequent activation of caspase-8, promoting caspase-3 activation and the DNA fragmentation in DCs. A2. Modulation of apoptosis through the intrinsic pathway in DCs infected with HSVs. HSV-1 infection generates the up-regulation of p53, a protein that activates Bcl-2-associated X protein (BAX). Once BAX is activated, its dimerization in the mitochondrial outer membrane promotes mitochondrial membrane permeabilization and the release of cytochrome c (Cytc). Cyt c plays an essential role in the activation of caspase-9, allowing caspase-3 activation for inducing DNA fragmentation. B. Regulation of autophagy in DCs infected with HSVs. HSV-1 induces an increase in the expression of proteins associated with autophagosome formation, such as microtubule-associated protein-1 light chain 3 (LC3-I) and LC3-II formation. However, autophagosome formation is inhibited by the γ134.5 viral protein. Moreover, the maturation of autophagosomes which consists of their fusion with lysosomes is inhibited by HSV-1 and HSV-2 infection in DCs. C. Modulation of the unfolded protein response (UPR) in DCs infected with HSV. The UPR response is regulated by three sensors called inositol-requiring protein 1α (IRE-1α), protein kinase RNA (PKR)-like ER kinase (PERK), and the activating transcription factor 6 (ATF6). IRE-1α is located in the endoplasmic reticulum (ER) and catalyzes the non-canonical splicing of X-box binding protein 1 (XBP-1) mRNA. HSV-2 infection modulates XBP-1, producing an increase in its splicing in DCs. Conversely, HSV-2 infection in DCs does not modulate the PERK and ATF6 sensors
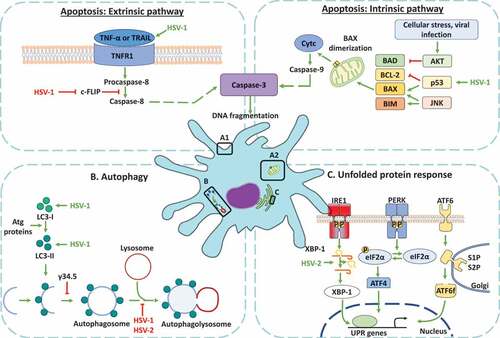
Table 2. Attenuated herpes simplex virus vaccine candidates that show favorable interactions with DCs
Data availability statement
Data sharing does not apply to this article as no new data were created or analyzed in this review.
