Figures & data
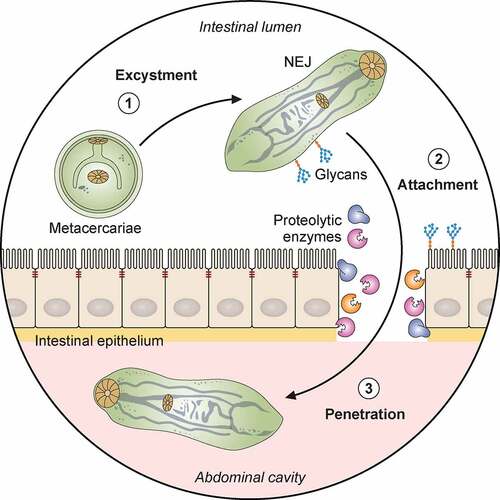
Figure 1. Pathogenicity of F. hepatica within the mammalian host. 1: The metacercariae of F. hepatica become activated by a series of stimuli (CO2, temperature, bile salts, reducing conditions, pH) as they pass through the digestive system of a mammalian host. 2: The metacercariae excyst in the small intestine releasing NEJs that attach to the gut wall via surface glycans and penetrate through the intestinal epithelia with the aid of secreted cathepsin peptidases. 3: Once in the abdominal cavity, and throughout its lifecycle, F. hepatica expresses a plethora of virulence factors that enable the parasite to evade and modulate the host’s immune response. Many of these factors (Cathepsins; Fatty acid binding proteins, FABP; Helminth defense molecule, FhHDM; Extracellular vesicles, EVs) hamper the activation of host immune cells by limiting their ability to respond to inflammatory stimuli and subsequent capacity to promote antigen specific Th1/Th17- responses that are required to effectively clear infection. 4: In contrast, secreted proteins (Peroxiredoxin, FhPrx; parasite glycoproteins), as well as glycoconjugates on the tegmental surface of the parasite, actively recruit and modulate dendritic cells and M2 macrophages which favor the induction of Th2/regulatory immune responses, creating an immunological environment that benefits the parasites survival. 5: Over a period of days, the NEJs migrate through the abdominal cavity to the liver, where they begin tunneling a path through the connective tissue of the parenchyma, facilitated by parasite secreted cathepsin peptidases (FhCL2, FhCL3) capable of degrading the liver extracellular matrix. 6 and 7: The extensive tissue damage caused by the migration through the liver initiates a wound healing response characterized by the influx of immune cells, and subsequent induction of fibrosis to repair the damage. 8: Flukes reach the biliary ducts of the mammalian host approximately 12 weeks after infection. 9: Blood is a vital nutrient source for the mature parasites in the bile ducts, and they express several proteins related to red blood cell lysis, hemoglobin digestion and metabolism (Saposins, Cathepsins, Aminopeptidases). 10: The acquisition of these extra nutrients from blood allows Fasciola to produce thousands of eggs which are shed via the host’s feces that results in the infection of the intermediary snail host, restarting the parasite lifecycle. Adapted from different templates available in BioRender.com (2021). Retrieved from https://app.biorender.com/biorender-templates
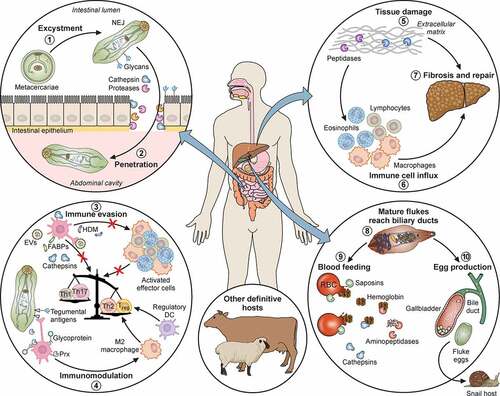
Figure 2. Life stages of F. hepatica and F. gigantica. A: Liver from an infected sheep with acute F. hepatica infection (reported by López Corrales et al. [Citation157]) showing gross pathology. The white tracks delineate the tunneling activity of the parasites through the liver tissues, most commonly observed on the left lobe of liver closest to the intestine in situ (LL). GB: Gall bladder. Scale bar, 1 cm. B: Microscopical liver pathology shown by hematoxylin and eosin (HE) stained serial liver sections from a mouse infected with F. hepatica (Molina-Hernandez and Dalton, unpublished). Top panel: Liver section displaying the migratory tracts (white arrows) formed by the invading F. hepatica immature flukes (black arrow). Bottom panel: The damage caused by the migrating parasite (black arrow) is resolved with no visible tracts in the liver and acute necrotic foci (ne) comprised of inflammatory cells (mainly eosinophils and macrophages). Scale bar, 200 µM. C: Adult F. gigantica (Fg) and F. hepatica (Fh) parasites. Note the typical leaf shape morphology of each species and the size variation. The length-to-width ratio of adult F. gigantica is greater than that of F. hepatica parasites. F. hepatica parasites have a broader anterior end, with defined shoulders, whilst F. gigantica is narrower and lacks this definition. Scale bar, 1 cm. D: The differential morphology of eggs from F. hepatica (white arrows) and F. gigantica (black arrows). The eggs of F. gigantica are typically larger, but variation exists in different definitive hosts and thus a considerable overlap is observed. Scale bar, 100 µM. E: The invasive newly excysted juvenile stage of F. hepatica has a typical cephalic cone shape. Antibodies to the digestive cathepsin peptidase L3 were used to probe the parasite and highlights their bifurcated gut (g) represented by the green fluorescence. The musculature of the parasite is highlighted by the red fluorescence, that accentuates the oral sucker (OS), ventral sucker (VS) and tegument of the parasite. Scale bar, 20 µM
![Figure 2. Life stages of F. hepatica and F. gigantica. A: Liver from an infected sheep with acute F. hepatica infection (reported by López Corrales et al. [Citation157]) showing gross pathology. The white tracks delineate the tunneling activity of the parasites through the liver tissues, most commonly observed on the left lobe of liver closest to the intestine in situ (LL). GB: Gall bladder. Scale bar, 1 cm. B: Microscopical liver pathology shown by hematoxylin and eosin (HE) stained serial liver sections from a mouse infected with F. hepatica (Molina-Hernandez and Dalton, unpublished). Top panel: Liver section displaying the migratory tracts (white arrows) formed by the invading F. hepatica immature flukes (black arrow). Bottom panel: The damage caused by the migrating parasite (black arrow) is resolved with no visible tracts in the liver and acute necrotic foci (ne) comprised of inflammatory cells (mainly eosinophils and macrophages). Scale bar, 200 µM. C: Adult F. gigantica (Fg) and F. hepatica (Fh) parasites. Note the typical leaf shape morphology of each species and the size variation. The length-to-width ratio of adult F. gigantica is greater than that of F. hepatica parasites. F. hepatica parasites have a broader anterior end, with defined shoulders, whilst F. gigantica is narrower and lacks this definition. Scale bar, 1 cm. D: The differential morphology of eggs from F. hepatica (white arrows) and F. gigantica (black arrows). The eggs of F. gigantica are typically larger, but variation exists in different definitive hosts and thus a considerable overlap is observed. Scale bar, 100 µM. E: The invasive newly excysted juvenile stage of F. hepatica has a typical cephalic cone shape. Antibodies to the digestive cathepsin peptidase L3 were used to probe the parasite and highlights their bifurcated gut (g) represented by the green fluorescence. The musculature of the parasite is highlighted by the red fluorescence, that accentuates the oral sucker (OS), ventral sucker (VS) and tegument of the parasite. Scale bar, 20 µM](/cms/asset/1d730547-b162-4d52-9354-0004ef1a7d9e/kvir_a_1996520_f0002_oc.jpg)
Table 1. Major Species of snails from the Family Lymnaeidae infected with F. hepatica globally
Figure 3. The profile of proteins secreted by the three main life cycle stages migrating through the host. The proteomic data for the newly excysted juveniles 24 h post-excystment (NEJ 24), the immature fluke 21 days post-infection (Immature) and the adult parasites (Adult) is extrapolated from Cwiklinski et al. [Citation13], Cwiklinski et al. [Citation197] and Murphy et al. [Citation183], respectively. The protein abundance across the three stages, represented by the emPAI values detailed, is highlighted from low to high abundance by the yellow to green color scale, respectively. Proteins that are secreted in multiple isoforms, such as the cathepsin L peptidases, have been grouped together. The 12 uncharacterized proteins are shown as one group. *Abbreviated name for 4-methyl-5(B-hydroxyethyl)-thiazole monophosphate biosynthesis enzyme
![Figure 3. The profile of proteins secreted by the three main life cycle stages migrating through the host. The proteomic data for the newly excysted juveniles 24 h post-excystment (NEJ 24), the immature fluke 21 days post-infection (Immature) and the adult parasites (Adult) is extrapolated from Cwiklinski et al. [Citation13], Cwiklinski et al. [Citation197] and Murphy et al. [Citation183], respectively. The protein abundance across the three stages, represented by the emPAI values detailed, is highlighted from low to high abundance by the yellow to green color scale, respectively. Proteins that are secreted in multiple isoforms, such as the cathepsin L peptidases, have been grouped together. The 12 uncharacterized proteins are shown as one group. *Abbreviated name for 4-methyl-5(B-hydroxyethyl)-thiazole monophosphate biosynthesis enzyme](/cms/asset/ee31dad1-547c-47d9-adab-ee96adec17b4/kvir_a_1996520_f0003_oc.jpg)
Data Availability
Data sharing is not applicable to this article as no new data were created or analysed in this study.
