Figures & data
Figure 1. Overview of the domains of neisserial heparin-binding antigen (NHBA), neisserial IgA protease (IgAp), and the B. pertussis filamentous hemagglutinin precursor FhaB (not drawn to scale). Sequence data refer to the proteins of N. meningitidis strain H44/76 and B. pertussis strain Tohama I. The domains and subdomains are differently colored. Approximate positions for processing sites are indicated with arrows, and the molecular mass of the resulting fragments is shown. The position and the sequences of the Arg-rich regions are depicted. hLF, human lactoferrin; PKLK, plasma kallikrein; TPS, two-partner secretion domain; MCD, mature C-terminal domain; CPD, carboxy proximal domain; SP, signal peptide
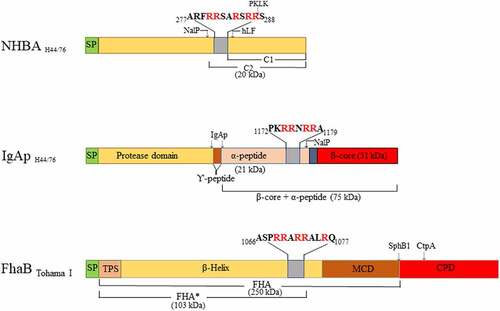
Table 1. Strains and plasmids used in this study
Figure 2. Effect of FCS on biofilm formation. (a, b) Impact of different concentrations of FCS in TSB on biofilm initiation of N. meningitidis (Nm) strains HB-1 (a) and its nalP mutant derivative (b). (c, d) Impact of the addition of 100 µg/ml of DNase I (c) or 10% FCS (d) in the culture medium on biofilm formation of H. influenzae (Hi) strain R2866, S. aureus (Sa) strain NCTC 8178, B. pertussis (Bp) strain B213, and N. gonorrhoeae (Ng) strains FA1090 and VG3. 1-h-old biofilms were generated for Nm, Ng 1090, Hi, and Sa, while 3-h-old and 12 h old biofilms were generated for Ng VG-3 and BpB213,respectively. The data represent the means and standard deviations of three independent experiments. Values are given as relative to no treatment (without DNase I or FCS), which was set at 100. Statistically significant differences between groups were determined with unpaired t-test and are marked with one (P < 0.05), two (P < 0.005), or three asterisks (P < 0.0005)
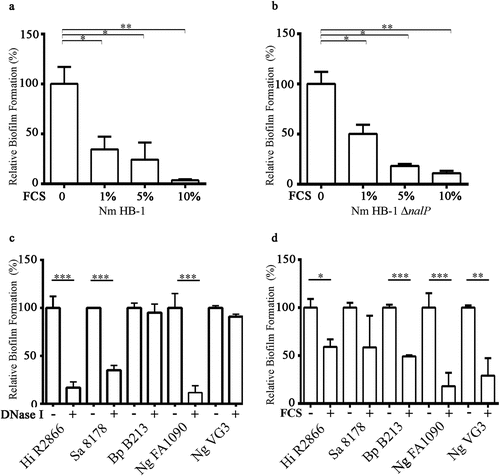
Figure 3. Impact of FCS on biofilm architecture. (a) Organization of 1- and 12-h-old biofilms of N. meningitidis strain HB-1 ΔnalP_GFP produced in TSB supplemented or not with 1% of FCS. (b) Biofilm parameters (biomass, thickness and roughness) were calculated using COMSTAT software. Values are means of data from at least five image stacks of two independent replicates and bars indicate standard deviation. Statistically significant differences between groups were calculated by unpaired t-test and marked with one asterisk (P < 0.05)
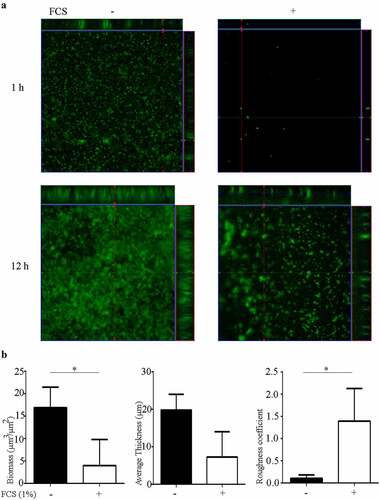
Figure 4. Far dot-blotting assay probing the binding of recombinant α-peptide of IgAp and NHBA in the presence or absence of FCS to DNA. Various amounts of the linearized plasmid pETx507_AutAp and, as a control, of 1000 ng of BSA were spotted onto a membrane. The membrane was then blocked and subsequently incubated with recombinant NHBA or α-peptide of IgAp, which were first incubated or not with FCS. The binding of the peptides to the DNA on the membrane was analyzed by immune detection using anti-α-peptide or anti-NHBA antibodies
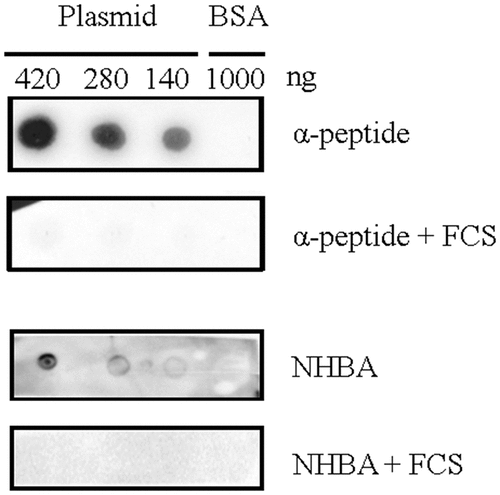
Figure 5. Cleavage of meningococcal proteins by serum proteases. (a) Cell envelope preparations of N. meningitidis strain HB-1 ΔnalP were incubated with 5 µg of PKLK, α-FXIIa, β-FXIIa, or plasmin. Cleavage of the IgAp α-peptide, fused via the linker to the TD, was analyzed by immunoblotting using antiserum against the TD . (b) 0.5 µg of the recombinant NHBA polypeptide was incubated with 8 or 80 ng of PKLK and with 0.032 or 0.32 ng of plasmin and analyzed by immunoblotting using a antiserum against NHBA
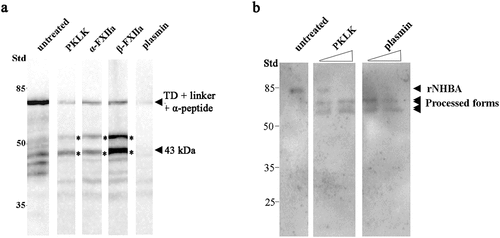
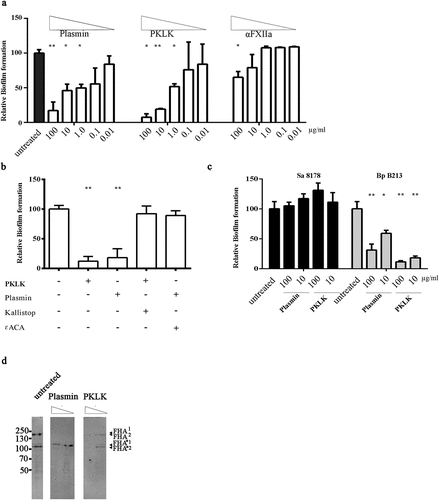
Figure 6. Inhibition of biofilm formation by serum proteases. (a) Biofilms of HB-1 ΔnalP were formed under static conditions in presence of the purified proteases at the different concentrations indicated. (b) Biofilms were studied of HB-1 ΔnalP in the presence of 100 of PKLK or 10 µg/ml of plasmin, respectively, which were either preincubated or not with 50 µM Kallistop or 200 mM ɛACA, respectively. (c) Impact of different concentrations of plasmin and PKLK on biofilm formation of S. aureus strain 8178 and B. pertussis strain B213. In panels a–c, values are given as relative to the no-treatment control, which was set at 100%. Statistically significant differences for each treatment relative to the untreated control are marked with one (P < 0.05) or two (P < 0.005) asterisks (unpaired t-test). (d) Whole cells of B. pertussis strain B213 were incubated with 8 or 80 ng of PKLK or with 0.032 or 0.32 ng of plasmin and analyzed by immunoblotting using anti-FHA monoclonal antibodies
Supplemental Material
Download PDF (746.8 KB)Data availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
