Figures & data
Figure 1. Commensal sites of C. albicans in the human body and clinical manifestations of C. albicans infection. Taking advantage of its commensal niches in the oral and genital mucosal surfaces and gastrointestinal tract, C. albicans can cause invasive disease (yellow star) and mucosal disease (blue star) in several tissues. Illustration created with BioRender.com.
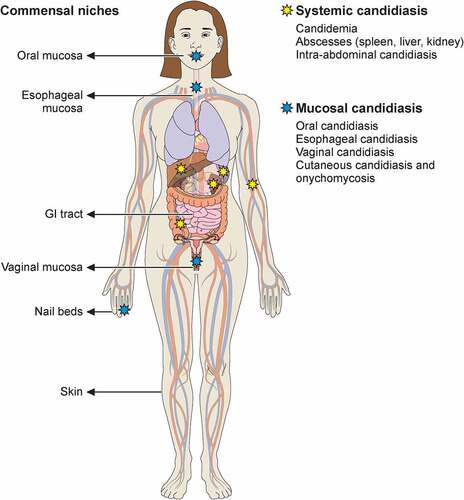
Table 1. Key C. albicans-associated virulence factors
Figure 2. Recognition of C. albicans by immune cells is mediated by distinct pattern recognition receptor signaling pathways. Extracellular recognition of fungal ligands occurs via C-lectin receptors (CLRs) or by some of the Toll-like receptors (TLRs) such as TLR1, TLR2, TLR6, or TLR4. Recognition leads to the activation of intracellular signaling pathways dependent on several adaptor molecules inducing NF-κB activation and cytokine secretion. In addition, CLRs can activate AP-1 via MAPK also leading to cytokine secretion. Some TLRs (TLR3, TLR7, TLR9) recognize nucleic acids derived from C. albicans within the endosome. Within the cytosol, recognition is mediated by NOD-like Receptors (NLRs) with resultant inflammasome activation and IL‐1β processing. Recognition by TLRs and CLRs and secretion of pro-IL‐1β and pro-IL-18 triggers inflammasome assembly, activation of pro-caspase 1 to generate caspase-1, and cleavage of these two cytokines into their mature IL‐1β and IL-18 forms. Additional cytosolic recognition may occur via RIG-I-like receptors. Illustration created with BioRender.com. TLR, Toll like receptor; MyD88, Myeloid differentiation factor 88; IRAK1, Interleukin 1 receptor associated kinase 1; TAK1, transforming growth factor-β-activated kinase 1; TRAF, Tumor necrosis factor receptor-associated factor; TAB, TGF-beta-activated kinase; NEMO, nuclear factor-κB essential modulator; IκB kinase; CLYD, cylindromatosis tumor suppressor; RLR, RIG-I-like receptor; CLR, C-lectin receptor; Mincle, macrophage inducible Ca2+-dependent lectin receptor; FcγR, Fc receptors: SYK, Spleen tyrosine kinase; ITAM, immunoreceptor tyrosine based activation motif; PLCγ2 Phospholipase C gamma 2; PKCδ, Protein kinase C delta; CARD9, caspase recruitment domain-containing protein 9; Malt1, mucosa-associated lymphoid tissue lymphoma translocation 1; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; DC-SIGN, Dendritic cell-specific intercellular adhesion molecule-3-Grabbing non-integrin; CR3, Complement receptor 3; MAPK, mitogen-activated protein kinase; ERK, Extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; AP-1, activator protein-1; ASC, Apoptosis-associated speck-like protein containing a CARD; NLRP3, NLR family pyrin domain containing 3; NLRC4, NLR family CARD domain containing 4.
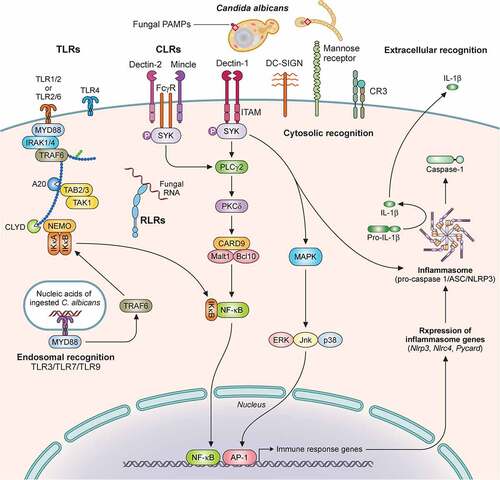
Table 2. Inborn errors of immunity underlying inherited susceptibility to C. albicans infections
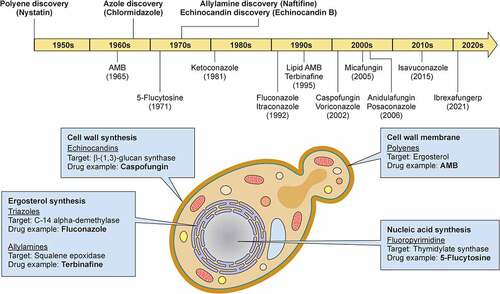
Figure 3. Milestones in antifungal drug development and fungal targets of the currently available antifungal agents. In the upper panel, the timeline depicts the date of discovery of the first indicated antifungal compound within each class of antifungal drugs and the date of FDA approval for the most common antifungal drugs with anti-Candida activity. In the lower panel, a C. albicans budding yeast is depicted and the targets of antifungal drugs are shown. Illustration created with BioRender.com. AMB, amphotericin B.
Data availability statement
No data sets were analyzed in this manuscript, and data availability is not applicable.
