Figures & data
Figure 1. Permeability of ARS in bacterial species.
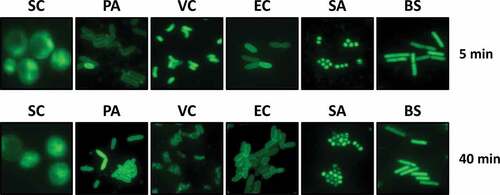
Figure 2. Antibacterial efficacy of ARS in vivo and in vitro.
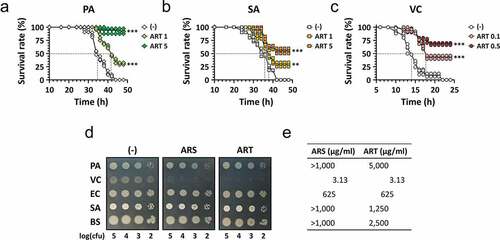
Figure 3. Enhancement of ARS-induced ROS generation in VC.
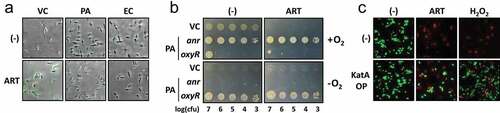
Figure 4. Enhancement of ARS-induced DNA damage in VC.
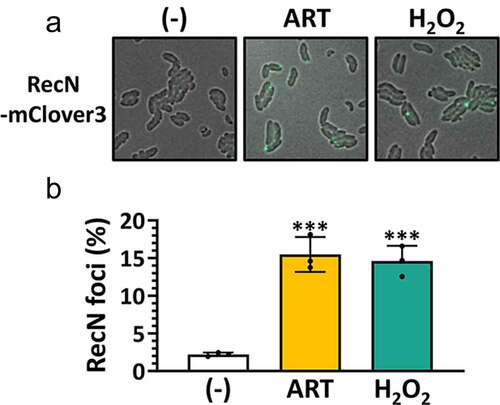
Figure 5. Enhanced antibacterial activity of ARS by copper addition.
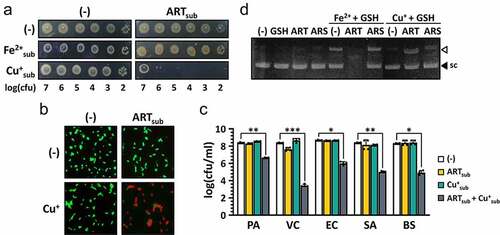
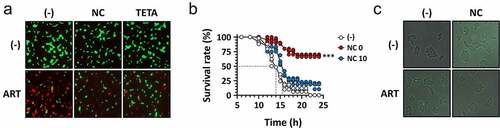
Figure 6. Reduced antibacterial activity of ARS by copper chelators.
Supplemental Material
Download Zip (755.8 KB)Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request ([email protected]).
