Figures & data
Table 1. Current understanding of glycosylation of viral proteins
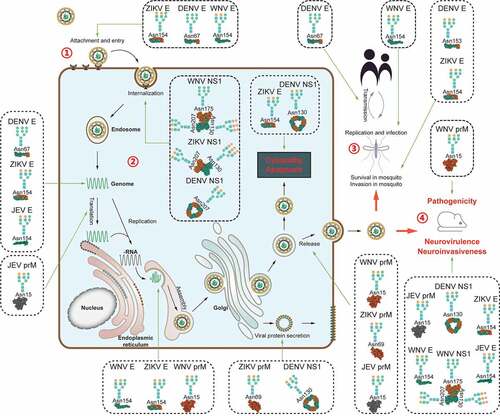
Figure 1. Diverse functions of glycosylation of viral proteins from mosquito-borne flaviviruses.
Data availability statement
Data sharing not applicable to this article as no data sets were generated or analyzed during the current study. Data cited in this review are published and available online or upon request from the authors of the respective publications.
